ISSN: 2206-7418
Nanotheranostics 2018; 2(4):360-370. doi:10.7150/ntno.27142 This issue Cite
Research Paper
In vivo therapeutic evaluation of polymeric nanomedicines: effect of different targeting peptides on therapeutic efficacy against breast cancer
Centre for Advanced Imaging, Australian Institute for Bioengineering and Nanotechnology and ARC Centre of Excellence in Convergent Bio-Nano Science and Technology, University of Queensland, Brisbane, 4072, Australia
QIMR Berghofer Medical Research Institute, 300 Herston Road, Brisbane, QLD, 4006, Australia
Abstract
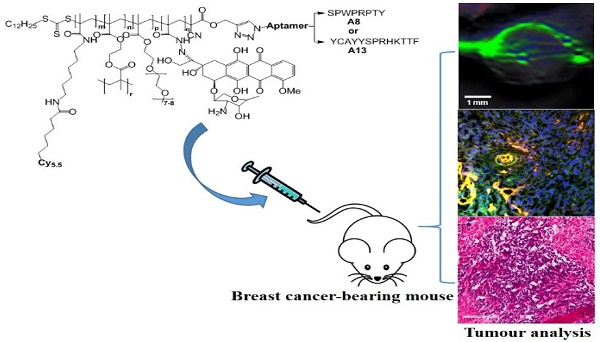
Targeted nanomedicines offer many advantages over macromolecular therapeutics that rely only on passive accumulation within the tumour environment. The aim of this work was to investigate the in vivo anticancer efficiency of polymeric nanomedicines that were conjugated with peptide aptamers that show high affinity for receptors on many cancer cells. In order to assess the ability for the nanomedicine to treat cancer and investigate how structure affected the behavior of the nanomedicine, three imaging modalities were utilized, including in vivo optical imaging, multispectral optoacoustic tomography (MSOT) and ex vivo confocal microscopy. An 8-mer (A8) or 13-mer (A13) peptide aptamer that have been shown to exhibit high affinity for heat shock protein 70 (HSP70) was covalently-bound to hyperbranched polymer (HBP) nanoparticles with the purpose of both cellular targeting, as well as the potential to impart some level of chemo-sensitization to the cells. Furthermore, doxorubicin was bound to the polymeric carrier as the anticancer drug, and Cyanine-5.5 (Cy5.5) was incorporated into the polymer as a monomeric fluorophore to aid in monitoring the behavior of the nanomedicine. Enhanced tumour regression was observed in nude mice bearing MDA-MB-468 xenografts when the nanocarriers were targeted using the peptide ligands, compared to control groups treated with free DOX or HBP without aptamer. The accumulated DOX level in solid tumours was 5.5 times higher in mice treated with the targeted therapeutic, than mice treated with free DOX, and 2.6 times higher than the untargeted nanomedicine that relied only on passive accumulation. The results suggest that aptamer-targeted therapeutics have great potential for improving accumulation of nanomedicines in tumours for therapy.
Keywords: peptide aptamers, chemo-sensitization, Multispectral Optoacoustic Tomography (MSOT), nanomedicine

 Global reach, higher impact
Global reach, higher impact