ISSN: 2206-7418Nanotheranostics
Nanotheranostics 2024; 8(1):90-99. doi:10.7150/ntno.88849 This issue Cite
Review
Drug delivery for neurodegenerative diseases is a problem, but lipid nanocarriers could provide the answer
1. Department of Neuroscience, City University of Hong Kong, Kowloon, Hong Kong SAR, China.
2. Department of Pharmacy, Jagannath University, Dhaka-1100, Bangladesh.
Abstract
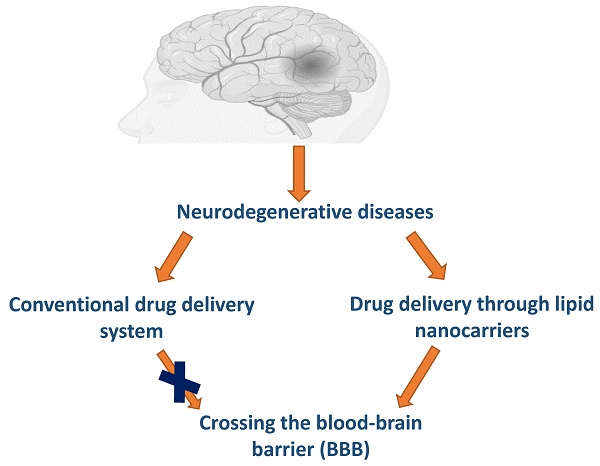
Neurodegenerative disorders encompass diseases that involve the degeneration of neurons, particularly those within the central nervous system. These are the most commonly observed disorders among the geriatric population. The treatment or management of this condition presents additional challenges due to therapeutics that may not be as effective as desired. The primary obstacle that often hinders the efficacy of therapy is the existence of a blood-brain barrier (BBB). The BBB serves as a vital safeguard for the brain, effectively obstructing the passage of drugs into the brain cells. Hence, the management of damaging neurodegenerative conditions such as Alzheimer's disease (AD), Parkinson's disease (PD), Cerebrovascular diseases (CVDs), Huntington's disease (HD), and Multiple sclerosis (MS) is currently the primary area of research interest. The innovative utilization of nanoparticles as drug carriers provides renewed optimism in addressing many complicated medical conditions. In this article, I have aimed to gather published information regarding various lipid nanoparticles that can efficiently transport medication to the brain to address neurodegenerative disorders. According to the published literature, liposomes, solid-lipid nanoparticles, nanostructured nanoparticles, microemulsions, and nanoemulsions are potential nanocarriers that can treat neurodegenerative disorders.
Keywords: nanoparticles, neurodegenerative disorders, blood-brain barrier, nanocarriers, central nervous system (CNS)
1. Introduction
To function and transmit electrical signals efficiently, the neurons of the CNS need a constant supply of nutrients and other gases [1]. To maintain a perfect brain environment, the fluids surrounding neurons and shielding them from mechanical disturbances must be tightly regulated [2]. Brain cells are not only protected by fluids but also by the blood-brain barrier (BBB). The physical barrier known as the BBB comprises specialized endothelial cells, astrocytes, pericytes, and neurons that keep the brain in a state of homeostasis by closely maintaining the flow of chemicals into the CNS [3].
The generation of neurodegenerative disorders such as Alzheimer's disease (AD), Parkinson's disease (PD), Huntington's disease (HD), Multiple sclerosis (MS), or Amyotrophic lateral sclerosis is facilitated by pathogenic processes that affect the CNS [4]. To protect the brain from toxic and poisonous substances blood-brain barrier is necessary, but it also serves as a barrier to drug entry due to its higher selectivity [4]. The discovery of novel medications for CNS disorders, the BBB, poses a significant limitation. Certain chemicals cannot pass the barrier to enter the brain. Peptides, recombinant proteins, monoclonal antibodies, RNA interference (RNAi)-based medications, and gene therapy drugs are large molecules that usually do not penetrate the BBB [5].
The literature reports several methods to administer medications to the brain, including intranasal administration and intracerebroventricular and intracerebral administrations, to pass the BBB in response to different stimuli [6]. High drug loading, improved physical and chemical stability, and low toxicity of carrier molecules should all be considered when building a drug delivery system [7]. The main problem is creating a model that maintains the BBB's basic properties while remaining compatible with the drug's effectiveness [8]. The most effective way to get the right medications into the CNS is still unclear for many disorders [9]. Various nanocarriers have been produced and used as CNS delivery systems for diagnostic and therapeutic reasons, enhancing biological distribution and pharmacokinetics and raising drug concentration in the brain [10]. Due to their unique optical, thermal, magnetic, and physicochemical characteristics, such as small size, large surface area, strong macromolecule adsorption capacity, and high chemical reactivity, materials with mean sizes ranging from 0.1 nm to 100 nm aim to cross the BBB have drawn attention [11, 12].
Nanomedicines are expected to help drugs pass the BBB, boosting their bioavailability based on their superior qualities [12]. However, polymeric nanoparticles are not as effective as lipid nanocarriers. The utilization of lipid nanoparticles presents a promising avenue for overcoming the obstacles commonly encountered with polymeric nanoparticles, including the undesirable cytotoxic effects and the absence of viable approaches for efficient large-scale manufacturing [13, 14]. Lipid-based novel drug delivery systems have been primarily centered around transporting lipophilic molecules. Nonetheless, there has been a recent surge of interest in lipoid drug delivery systems, primarily due to their fundamental characteristics such as biocompatibility, self-assembly capabilities, capacity to traverse the BBB, variability in particle size, and cost-effectiveness. These qualities render lipid-based delivery systems considerably more appealing [15, 16]. Due to their efficiency in encapsulating and crossing biological membranes to carry lipophilic drugs, lipid nanoparticles, such as solid lipid nanoparticles (SLNs), liposomes, nanoemulsion, and nanostructured lipid carriers (NLCs), are the most promising drug delivery systems that have been suggested by researches [17-19]. Specifically, SLNs have gained attention as a potential drug delivery system that has the potential to deliver drugs to the specific target site of the brain after passing BBB effectively. This new strategy offers controlled drug delivery, a longer circulation time, higher target specificity, and efficacy [20].
This review aimed to incorporate research advances in developing lipid-based nanocarrier therapeutics for their implications in treating neurodegenerative disorders.
2. Drug Administration Complexities Due to BBB
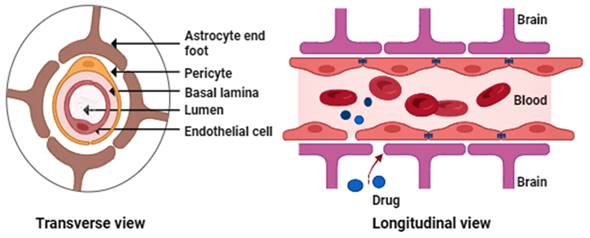
The BBB is essential to the body's neurovascular system, communicating with the CNS. It is well known that the BBB is the primary impediment to properly treating neurological disorders because it limits the CNS from receiving a variety of potentially helpful therapeutic and diagnostic substances. It also limits the free flow of chemicals between brain cells [21]. The major BBB constituents (Figure 1) include endothelial cells, astrocytic end-foot connections, basal lamina, tight junctions, and pericytes [22-23].
Preventing toxic substances from entering the brain is possible by maintaining peripheral circulation from the CNS [25]. Through passive transport, the BBB performs filtering tasks and selectively permits the passage of substances, including water, nutrients, and hydrophobic compounds [26]. The blood-cerebrospinal fluid barrier (BCSFB) adds another barrier to treatments and increases CNS complexity. The choroid plexus is a collection of ependymal cells that also serve as a barrier in the brain by isolating blood from cerebrospinal fluid (CSF) [27]. The BBB and other efflux transporters are crucial in ineffective drug absorption into the brain. The BBB has many ATP-dependent efflux transporters [25]. BBB's active efflux systems and overexpressed tight junctions also prevent medicines from reaching their therapeutic target. P-glycoprotein (P-gp), an ABC transporter, efficiently removes drugs from the brain and pumps them back into circulation [28].
3. Neurodegenerative Disorders
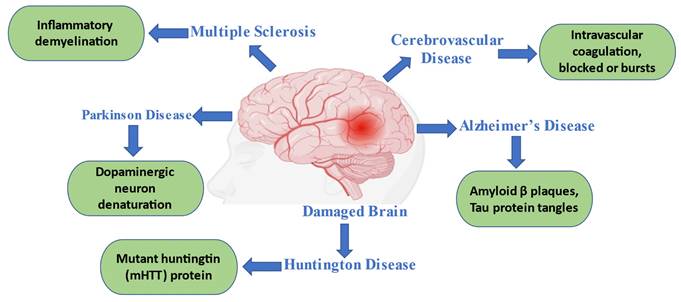
Age-related neurodegenerative diseases (NDs) are widespread. Although the peripheral neurological system (PNS) is also affected, the CNS is affected the most among the aging population [29]. Neuronal brain and spinal cord loss are a hallmark of many diseases. Alzheimer's, Parkinson's, Huntington's illnesses, multiple sclerosis, and amyotrophic lateral sclerosis are a few examples of neurodegenerative diseases (Figure 2). Clinical characteristics of each disease vary depending on the CNS regions implicated; some, for example, cause cognitive and memory problems, while others impair speech, locomotion, and breathing abilities. The development of novel and more potent treatments, which are desperately needed, can only be sped up by a greater understanding of the pathophysiology and pathogenesis of diseases [9, 30].
Generalized figure of the blood-brain barrier physiology.
3.1 Alzheimer's Disease (AD)
The primary cause of dementia in late adulthood is Alzheimer's disease, which is recognized as a progressive, multifaceted neurological condition [31]. Alois Alzheimer first introduced the concept of AD in 1906. Amyloid- β is deposited extracellularly as plaques, hyperphosphorylated tau protein aggregates intracellularly as tangles, and intra-cortical projecting neurons gradually degenerate in AD [32]. Acetylcholinesterase inhibitors and N-methyl-D-aspartate receptor antagonists are two therapy options [33]. New tactics have been devised to alter the illness process. Aβ and tau-based therapies are currently the focus of significant research and development in this area, and they hold the key to curing this disease in the near future [34, 35].
3.2 Parkinson's Disease (PD)
Dopaminergic neuron denaturation presence in substantia nigra causes striatal dopamine reduction in PD, the second most prevalent neurodegenerative illness [36, 37]. The condition progresses with a gradual loss of motor control, which causes severe respiratory and gastrointestinal issues that ultimately result in the patient's death [38]. Although the cornerstone of PD treatment is dopamine supplementation, the acetylcholine, norepinephrine, and serotonin systems are also found to be defective in Parkinson's disease [39-43]. Effective preliminary treatments include levodopa preparations, dopamine agonists, and monoamine oxidase-B (MAO-B) inhibitors. Anticholinergic medications, such as trihexyphenidyl, are helpful for young people with severe tremors. Still, they should be used with caution due to the possibility of side effects, particularly those that could affect cognition [44].
3.3 Cerebrovascular Diseases (CVDs)
All illnesses that predominantly affect the brain's blood arteries are called cerebrovascular disorders [45]. The most frequent symptom of cerebrovascular condition is stroke. It happens when a cerebral artery becomes blocked or bursts [46]. There are two major types of strokes: ischemic stroke, which involves occlusions of cerebral vessels, and hemorrhagic stroke, which involves intracerebral hemorrhage (ICH) [47]. Hemorrhagic stroke can be brought on by chemotherapeutic side effects, cancer-related coagulopathies, particularly those from leukemia, and metastatic brain illness. Additionally, ischemic stroke can result from chemotherapy, non-bacterial thrombotic endocarditis (NBTE), a pro-thrombotic condition such as disseminated intravascular coagulation (DIC), or metastatic illness with local vessel invasion [48].
3.4 Multiple Sclerosis (MS)
MS usually affects young people and causes non-traumatic debilitating conditions [49]. MS develops gradually, affecting the spinal cord, brain stem, basal ganglia, visual neurons, and other CNS white matter [50]. An individual's genetic makeup, Epstein-Barr virus, sunlight, smoking, and vitamin D play significant roles in MS development [51]. Disease-modifying medicines and symptomatic therapy are both used to treat the symptoms of MS, which are brought on by neurological problems [49]. Interferons, glatiramer acetate, teriflunomide, sphingosine 1-phosphate receptor modulators, fumarates, cladribine, and three different kinds of monoclonal antibodies are commonly used for the treatment of MS [52].
3.5 Huntington's Disease (HD)
Dementia, behavioral and psychological issues, and uncontrollable choreatic movements indicate Huntington's disease (HD), a relatively uncommon neurodegenerative disease [53, 54]. George Huntington originally described HD, also known as hereditary chorea, in 1872. He talked about the genetic origin of the condition, how the disease occurs in individuals between the ages of 30 and 40, and psychological and cognitive symptoms [55]. Chromosome 4 has the huntingtin (HTT) gene, which increases the CAG trinucleotide repeats and causes HD. It causes the development of a mutant huntingtin (mHTT) protein having an extensive polyglutamine repeat [56]. The symptoms they address can help to categorize the current HD treatments. These categories include non-drug therapy, antipsychotic drugs, antidepressants, mood stabilizers, and chorea medications [57].
Common neurodegenerative disorders with their pathophysiological indications.
4. Applications of Nanoparticles (NPs) to Cross the BBB
The chemistry, design, and characteristics of the NPs dictate the possible method of NP-mediated drug transport through the BBB [58]. Since they have a small size and high surface-to-volume ratio, NPs make excellent candidates for use as drug carriers. The medications they carry can be released more quickly and have higher bioavailability because they are closer to the surface [59]. To deliver loaded pharmaceuticals to the desired site of action with improved release kinetics and increased therapeutic efficacy with maximum therapeutic benefits, precisely modified nanocarriers are required [60-62].
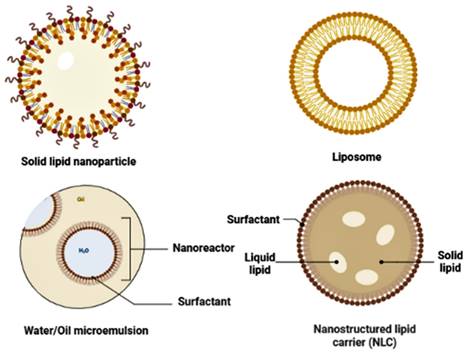
It should be emphasized that non-modified NPs administered systemically frequently interact with serum proteins unintendedly. In the reticuloendothelial system (RES), which is present mainly in the liver and spleen, opsonin adsorption on the surface creates a macrostructure known as a "corona," and this corona reported to be easily absorbed and destroyed by macrophages [63]. Coating NPs with hydrophilic polymers or surfactants is a commonly used method for resolving this problem; amphipathic polyethylene glycols have received much interest in investigating this topic [64, 65]. Nanocarriers can be made from various substances, such as carbon nanotubes, liposomes, micelles, polymeric and lipid-based nanoparticles (Figure 3), dendrimers, and micelles [66].
5. Lipid-based Nanocarriers
5.1 Solid Lipid Nanocarriers (SLNs)
Phospholipids are a crucial part of lipid and lipid-based drug delivery systems due to their many properties, including amphiphilic behavior, biocompatibility, and multi-functionality. The multiple drawbacks of liposomes, lipospheres, and microemulsion carrier systems, such as their difficult large-scale fabrication, poor percentage entrapment efficiency (% EE), and hard production process, have led to the development of the SLN delivery system [67-69]. When making SLN, solid lipids at body and room temperatures are often used, and they are stabilized using one or more surfactants [70]. The lipids used can be waxes, combinations of complicated glycerides, or highly purified triglycerides [70].
5.2 Liposome
In intranasal delivery, particularly for neurological diseases, liposomes-artificial lipid-based bilayered vesicles have become one of the most effective and popular lipid nanocarriers [71]. Typically, liposomal preparation comprises dissolving cholesterol, lecithin, and occasionally charge-inducing agent(s) in an organic solvent before drying to create a thin film. A liposome suspension is then made by dispersing the film in an aqueous solution at a crucial hydration temperature [72]. According to lipophilic properties, liposomal nanocarriers' most significant characteristic is protection from degradation and optimization of the pharmacokinetics of the encapsulated drug. These nanocarriers also release their content after phagocytosis and nonphagocytic endocytosis, which enables encapsulated drugs to enter the brain [73].
5.3 Nanostructured Lipid Nanocarriers (NLCs)
Similar to SLN in terms of physicochemical characteristics, NLC was created by nanostructuring the architecture of the lipid matrix to increase API loading while also preventing drug leakage during storage, giving them more flexibility to target particular API release profiles [74,75]. A second generation of lipid nanoparticles called NLCs was created to enhance drug loading [76]. NLCs are binary systems that contain solid and liquid lipids, which leads to a less organized lipidic core [77]. Although both SLNs and NLCs are useful for increasing the solubility of drugs in aqueous solutions and aiming for targeted drug release in the brain to treat various neurological disorders, the production process of NLCs and SLNs is the same. However, there are notable differences between the two dosage forms that only affect overall performances and the fate of such systems due to the types (nature) of lipids used to form their Matrigel [78].
5.4 Microemulsions (MEs) and Nanoemulsions (NEs)
An optically isotropic, thermodynamically stable system with two phases—an aqueous phase and a lipid phase- is called a microemulsion (ME) [79, 80]. The biphasic dispersion of two incompatible liquids, either oil in water (O/W) or water in oil (W/O) droplets stabilized by an amphiphilic surfactant, is what constitutes nanoemulsion (NE) [77]. There are some differences between the two sorts of systems. NEs are non-equilibrium, thermodynamically unstable systems as opposed to MEs [82, 83]. To increase stability, delivery, and bioactivity, numerous synthetic and natural chemicals have been produced. Nanoemulsions and microemulsions offer a good choice for the administration of drugs through lipophilic barriers [84].
6. Treatment of Common Neurodegenerative Diseases with Lipid Nanocarriers (Table 1)
6.1 Alzheimer's Disease
According to Dhawan et al., tween 80 and compritol were used to make SLN, which then encapsulated quercetin. This SLN-encapsulated quercetin gives good results in the aluminum-induced rats model to improve memory retention [85]. Quercetin-loaded nanosystems prepared by Pinheiro et al. show promise for treating neurological illnesses like AD, especially NLC, due to their improved capacity to transport quercetin to particular brain locations as well as their improved ability to inhibit amyloid- β aggregation [86].
Different types of lipid nanoparticles used for the effective delivery of drugs.
Lipid-based nanoformulations for the treatment of neurodegenerative disorders
| Drugs | Formulations | Diseases | References |
|---|---|---|---|
| Piperine | Solid lipid nanoformulation of piperine | AD | 109 |
| Quercetin | Quercetin-loaded solid lipid nanoparticles | AD | 86 |
| Phosphatidic acid | Phosphatidic acid or cardiolipin-loaded liposomes and solid lipid nanoparticles | AD | 110 |
| Curcumin | Curcumin-loaded solid lipid nanoparticles | CVDs | 111 |
| 3-n-Butylphthalide | 3-n-Butylphthalide (dl-NBP) in PEGylated-lipid nanoparticles (PLNs) in conjugation with Fas ligand antibody | CVDs | 112 |
| Edaravone | Edaravone-loaded lipid-based nanosystem (LNS) | CVDs | 113 |
| Rivastigmine | Solid lipid nanoparticle formulation of rivastigmine | AD | 114 |
| Tarenflurbil | The intranasal solid lipid nanoparticle of tarenflurbil | AD | 115 |
| Fibroblast growth factor (bFGF) | Gelatin-based nanostructured lipid carriers encapsulating fibroblast growth factor (bFGF) | PD | 116 |
| Apomorphine | Apomorphine-containing solid lipid nanoparticles (SLNs) with glyceryl monostearate (GMS) and polyethylene glycol monostearate (PMS) incorporated emulsifiers. | PD | 117 |
| Curcumin and piperine | Co-loading of curcumin and piperine in lipid-based nanoparticles | PD | 118 |
| Dimethyl fumarate (DMF) | lipid-based nanoparticles loaded with dimethyl fumarate (DMF) | MS | 102 |
| Phosphatidylserine and phosphatidic acid | Liposomes, prepared by phosphatidylserine and phosphatidic acid | MS | 119 |
| Pomegranate seed oil (PSO) | Nanoemulsion formulation of pomegranate seed oil | MS | 120 |
| Engeletin | Engeletin-nanostructured lipid nanocarriers | HD | 108 |
| CAG-siRNA | Lipid nanoparticles delivered cytosine-adenine-guanine (CAG)-trinucleotide-siRNA | HD | 121 |
Here, AD: Alzheimer's disease; CVDs: Cerebrovascular diseases; PD: Parkinson's disease; MS: Multiple sclerosis; HD: Huntington's disease
RVG29-nanoparticles, by Pinheiro et al., target the BBB and stimulate neuronal defense against amyloid-beta fibrillation [87]. It is a potentially effective way to distribute quercetin and a strategy that holds promise for Alzheimer's disease treatments in the future [87]. Findings from a study suggested that innovative EPO (erythropoietin)-loaded solid lipid nanoparticles may hold promise for treating neurodegenerative illnesses since they efficiently sustain memory in an animal model of AD [88].
A study's findings support a neuroinflammation model that mimics AD, in which inflammatory cells promote the synthesis of cytokines that cause inflammation while affecting learning and spatial memory. Intriguingly, MET-PSL (Metformin-loaded phosphatidylserine nanoliposomes) formulation may be more effective in treating AD in rats. It may suppress neuroinflammation markers like IL-1, TNF, and TGF-cytokines, improve neural tissue and cells, and restore learning and memory. The MET-PSL formulation may help AD patients with learning and memory problems and manage unchecked neuroinflammation [89].
Additionally, Jiang et al. showed intranasal transport of a Huperzine A-loaded lactoferrin-linked nanoemulsion to the brain. The hypothesized drug transport mechanism, receptor-mediated transcytosis, demonstrated the effectiveness of lactoferrin for brain targeting. Better pharmacokinetic and animal study data added credence to the idea that intranasal nanoemulsion would be helpful in AD site targeting [90].
6.2 Parkinson's Disease
In a study, hydrogel formulations containing RP (ropinirole) loaded lipid nanoparticles were successfully created and improved. Studies verified the RP's sustained and protracted release and its improved permeability. The lipid nanoparticles and hydrogel formulations showed enhanced oral and transdermal bioavailability upon PK studies. PCD studies demonstrate the repair of metabolic alterations in the rat PD model. The outcomes showed that SLN and NLC-enhanced hydrogel formulations could be considered alternative administration techniques to deliver RP for parkinsonism [91].
Uppuluri et al. designed and refined Piribedil (PBD) loaded SLNs. To facilitate effective intravenously administered drug administration, the optimized PBD-SLNs (Piribedil-loaded SLNs) were also loaded in Methyl Cellulose (MC)-based thermo-responsive in situ gels (PBD-SLN-ISG). Compared to intravenously administering a simple PBD suspension (PBD-Susp), the developed PBD-SLN-ISG demonstrated considerable nose-to-brain delivery with a 4-fold higher brain availability of PBD. These findings demonstrated the superiority of the created PBD-SLNs over the currently used traditional oral PBD therapy for treating Parkinson's disease [92].
In vivo, testing of Bacopa monnieri neuroprotective effects on rotenone-induced Parkinson's disease rats revealed that B. monnieri-SLNs loaded microneedle patches had superior neuroprotective action than pure drug [89]. The ROT (rotenone)-induced PD rodent model was used to assess the neuroprotective activity of naringenin-SLN in research by Mani et al. The findings of behavioral studies and biomarkers shed light on the idea that naringenin, an SLN, may have neuroprotective effects and potentially slow the course of PD [94]. Rahman et al. demonstrated that solid lipid nanoparticles with vitexin were effective against 6-hydroxydopamine-induced neurotoxicity in rats. It could be an additional, successful therapeutic strategy for managing PD [95].
6.3 Cerebrovascular Diseases
An experiment on brain distribution revealed that NLCs considerably improved the brain-targeting effectiveness of baicalein. In the central brain, NLCs effectively attack the cortex and brain stem. Vitamin E and gelucires may significantly impact pharmacokinetics and brain transport. It could be an efficient drug-targeting method for treating CNS illnesses and brain traumas because Tocol NLCs increase baicalein's stability and capacity to enter the brain [96]. In the stroke model, Ferulic acid (FA)-NLCs offer longer-lasting therapeutic effects by enhancing the pharmacological profile of FA. This nanoformulation may be a suitable controlled-release drug carrier system against ischemia injuries or other neurodegenerative disorders [97].
Gao et al. showed that compared to intravenously or orally administered daidzein suspension, the pharmacokinetic behavior of SLNs loading daidzein demonstrated that it may considerably lengthen circulation duration. Compared to oral suspension or intravenous solution, SLNs had a superior effect on the cardiovascular system of the anesthetized dogs. The SLNs outperformed the other two formulations regarding their ability to improve cerebral blood flow (CeBF) and lower cerebrovascular resistance (CeR) in sedated dogs and their ability to protect rats using an ischemia-reperfusion damage paradigm. These results suggest that SLNs loaded drugs may be a candidate for the therapy of cardio-cerebrovascular diseases. [98].
According to studies, the preferential accumulation of liposomes inside the lesion site is related to a biphasic pattern of BBB hyper-permeability. It offers a rare chance to transfer therapeutic molecules over the BBB selectively and effectively, a method that can be used for hemorrhagic stroke therapy [99]. All the symptomatic parameters of strokes of the MCAO (middle cerebral artery occlusion) rats were significantly improved by oleoylethanolamide (OEA) loaded LNPs, according to in vivo investigations. These findings strongly suggest that the produced nanoparticles will transform hydrophobic OEA into a possible therapy for stroke [100]. Sabry et al. claimed that a promising method for reducing the detrimental effects of stroke is the delivery of Valsartan to the brain using SLNs tagged with Rhodamine B [101].
6.4 Multiple Sclerosis
The findings of a study showed that DMF (Dimethyl Fumarate) loaded controlled release SLNs could be a promising choice for the enhancement of multiple sclerosis disease management [102]. Kumar et al. targeted treating neurological disorders like multiple sclerosis by using SLNs to orally transport methylthioadenosine (MTA) to the brain. The SLNs of stearic acid were smaller than 100 nm and provided more drug loading and entrapment. The pharmacokinetic studies' evidence for better bioavailability supported the pharmacodynamic findings. The investigations demonstrated the considerable brain delivery and efficient neuronal remyelination of SLN-encapsulated MTA. Additionally, they claimed that it might safely and efficiently treat MS-like symptoms [103].
Two axo-glial-glycoprotein antigens found in the Ranvier node, anti-Contactin-2 or anti-Neurofascin, were produced and used to surface-modify PEGylated SLNs in an investigation. In contrast to the control, when the surface was altered with anti-Contactin-2 or anti-Neurofascin; accordingly, their cellular absorption was increased by 4- and 8-fold. The findings of this study will aid scientists in creating MS nanocarriers that are more effective [104].
6.5 Huntington Disease
Dysfunctions in mitochondria play a significant role in the development of HD. Therefore, developing methodologies to treat mitochondrial defects may offer a future therapeutic approach. To treat 3-nitro-propionic acid (3-NP)-induced HD in rats, Sandhir et al. employed curcumin-encapsulated SLNs (C-SLNs). Comparing C-SLN-treated rats to 3-NP-treated rats, the C-SLN-treated rats' neuromotor coordination significantly improved, and other parameters like mitochondrial swelling, lipid peroxidation, protein carbonyls, and reactive oxygen species were significantly decreased. As a result, this study's findings imply that administering C-SLNs may be a practical therapeutic approach to treating HD [105].
Bhatt et al. successfully used heat homogenization to create SLNs that included Rosmarinic acid (RA). Treatment with RA-loaded SLNs can considerably lessen 3NP-induced deficiencies in body weight, beam walk, locomotor, motor coordination, and striatal oxidative stress. According to the results of this investigation, nasal injection of RA-loaded SLNs may be a potential strategy for managing HD [106].
The goal of Ramachandran & and Thangarajan was to compare thymoquinone solution (TQ-S) with solid lipid nanoparticles encapsulated with thymoquinone (TQ-SLNs) against 3-NP-induced behavioral despair, oxidative damage, and striatal pathology. The investigation's findings suggest that a moderate amount of TQ-SLNs dose is more than enough to achieve the effect of TQ-S to minimize behavioral, biochemical, and histological abnormalities in HD animals exposed to 3-NP [107]. Verma et al. suggested that Engeletin-nanostructured lipid nanocarriers can enhance transport and boost bioavailability when used to treat HD, which may result in treating or preventing this devastating condition [108].
7. Future Perspectives and Concluding Remarks
Research is an ongoing process, and research on neurodegenerative disorders is the most prominent topic for several sectors, like pathology, pharmacology, medicinal chemistry, drug discovery, and drug delivery sciences. Many people suffer from various neurodegenerative disorders, and scientists worldwide are trying to find effective therapeutics for those diseases. Since these diseases usually originate in the brain, we need to deliver drugs to the specific target site of the brain to treat the conditions, and most of the promising drug candidates fail to pass the blood-brain barriers. That is why scientists' focus is not only on discovering drugs but also on searching for an effective delivery system for drugs. In this aspect, nanoparticles shed some light on the hope of overcoming blood-brain barrier-related problems. Different research suggested that lipid nanoparticles can be a possible solution for the targeted delivery of drugs to treat neurodegenerative disorders. But, more extensive research on these fields is needed to validate their efficacy.
Acknowledgements
The images were drawn with the help of biorender.com. I am grateful to Professor Jufang He for the motivation to write this article.
Competing Interests
The author has declared that no competing interest exists.
References
1. Qiao R, Jia Q, Huwel S, Xia R, Liu T, Gao F, Galla HJ, Gao M. Receptor-mediated delivery of magnetic nanoparticles across the blood-brain barrier. ACS nano. 2012Apr24;6(4):3304-10
2. Hladky SB, Barrand MA. Mechanisms of fluid movement into, through and out of the brain: evaluation of the evidence. Fluids and Barriers of the CNS. 2014Dec;11:1-32
3. Pinheiro RG, Coutinho AJ, Pinheiro M, Neves AR. Nanoparticles for targeted brain drug delivery: what do we know?. International Journal of Molecular Sciences. 2021Oct28;22(21):11654
4. Teixeira MI, Lopes CM, Amaral MH, Costa PC. Current insights on lipid nanocarrier-assisted drug delivery in the treatment of neurodegenerative diseases. European journal of pharmaceutics and biopharmaceutics. 2020Apr1;149:192-217
5. Pardridge WM. Brain drug targeting: the future of brain drug development. Cambridge University Press. 2001 May 31
6. Li X, Tsibouklis J, Weng T, Zhang B, Yin G, Feng G, Cui Y, Savina IN, Mikhalovska LI, Sandeman SR, Howel CA. Nano carriers for drug transport across the blood-brain barrier. Journal of drug targeting. 2017Jan2;25(1):17-28
7. Patel M, Souto EB, Singh KK. Advances in brain drug targeting and delivery: limitations and challenges of solid lipid nanoparticles. Expert opinion on drug delivery. 2013Jul1;10(7):889-905
8. Bicker J, Alves G, Fortuna A, Falcão A. Blood-brain barrier models and their relevance for a successful development of CNS drug delivery systems: a review. European Journal of Pharmaceutics and Biopharmaceutics. 2014Aug1;87(3):409-32
9. Abbott NJ. Blood-brain barrier structure and function and the challenges for CNS drug delivery. Journal of inherited metabolic disease. 2013May;36:437-49
10. Naqvi S, Panghal A, Flora SJ. Nanotechnology: a promising approach for delivery of neuroprotective drugs. Frontiers in Neuroscience. 2020Jun9;14:494
11. Feng L, Wang H, Xue X. Recent progress of nanomedicine in the treatment of central nervous system diseases. Advanced Therapeutics. 2020May;3(5):1900159
12. Nguyen TT, Nguyen TT, Vo TK, Nguyen MK, Van Vo T, Van Vo G. Nanotechnology-based drug delivery for central nervous system disorders. Biomedicine & Pharmacotherapy. 2021Nov1;143:112117
13. Dhiman N, Awasthi R, Sharma B, Kharkwal H, Kulkarni GT. Lipid nanoparticles as carriers for bioactive delivery. Frontiers in chemistry. 2021Apr23;9:580118
14. Haider M, Abdin SM, Kamal L, Orive G. Nanostructured lipid carriers for delivery of chemotherapeutics: A review. Pharmaceutics. 2020Mar;12(3):288
15. Duan Y, Dhar A, Patel C, Khimani M, Neogi S, Sharma P, Kumar NS, Vekariya RL. A brief review on solid lipid nanoparticles: Part and parcel of contemporary drug delivery systems. RSC advances. 2020;10(45):26777-91
16. Salunkhe SS, Bhatia NM, Bhatia MS. Implications of formulation design on lipid-based nanostructured carrier system for drug delivery to brain. Drug Delivery. 2016May3;23(4):1306-16
17. Costa CP, Moreira JN, Lobo JM, Silva AC. Intranasal delivery of nanostructured lipid carriers, solid lipid nanoparticles and nanoemulsions: A current overview of in vivo studies. Acta Pharmaceutica Sinica B. 2021Apr1;11(4):925-40
18. Costa CP, Barreiro S, Moreira JN, Silva R, Almeida H, Sousa Lobo JM, Silva AC. In vitro studies on nasal formulations of nanostructured lipid carriers (NLC) and solid lipid nanoparticles (SLN). Pharmaceuticals. 2021Jul23;14(8):711
19. Costa C, Moreira JN, Amaral MH, Lobo JS, Silva AC. Nose-to-brain delivery of lipid-based nanosystems for epileptic seizures and anxiety crisis. Journal of Controlled Release. 2019Feb10;295:187-200
20. Satapathy MK, Yen TL, Jan JS, Tang RD, Wang JY, Taliyan R, Yang CH. Solid lipid nanoparticles (SLNs): an advanced drug delivery system targeting brain through BBB. Pharmaceutics. 2021Jul31;13(8):1183
21. Domínguez A, Suárez-Merino B, Goñi-de-Cerio F. Nanoparticles and blood-brain barrier: the key to central nervous system diseases. Journal of nanoscience and nanotechnology. 2014Jan1;14(1):766-79
22. Cecchelli R, Berezowski V, Lundquist S, Culot M, Renftel M, Dehouck MP, Fenart L. Modelling of the blood-brain barrier in drug discovery and development. Nature reviews Drug discovery. 2007Aug;6(8):650-61
23. Zhou J, Atsina KB, Himes BT, Strohbehn GW, Saltzman WM. Novel delivery strategies for glioblastoma. Cancer journal (Sudbury, Mass.). 2012 Jan;18(1)
24. Newton HB. Advances in strategies to improve drug delivery to brain tumors. Expert Review of Neurotherapeutics. 2006Oct1;6(10):1495-509
25. Witika BA, Poka MS, Demana PH, Matafwali SK, Melamane S, Malungelo Khamanga SM, Makoni PA. Lipid-Based Nanocarriers for Neurological Disorders: A Review of the State-of-the-Art and Therapeutic Success to Date. Pharmaceutics. 2022Apr11;14(4):836
26. Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiology of disease. 2010Jan1;37(1):13-25
27. Redzic ZB, Segal MB. The structure of the choroid plexus and the physiology of the choroid plexus epithelium. Advanced drug delivery reviews. 2004Oct14;56(12):1695-716
28. Omidi Y, Barar J. Impacts of blood-brain barrier in drug delivery and targeting of brain tumors. BioImpacts: BI. 2012;2(1):5
29. Kumar R, Aadil KR, Mondal K, Mishra YK, Oupicky D, Ramakrishna S, Kaushik A. Neurodegenerative disorders management: state-of-art and prospects of nano-biotechnology. Critical Reviews in Biotechnology. 2022Nov17;42(8):1180-212
30. Yacoubian TA. Neurodegenerative disorders: Why do we need new therapies?. InDrug discovery approaches for the treatment of neurodegenerative disorders. 2017 Jan 1 (pp. 1-16). Academic Press
31. Kumar A, Singh A. A review on Alzheimer's disease pathophysiology and its management: an update. Pharmacological reports. 2015Apr1;67(2):195-203
32. Dyrba M, Grothe MJ, Mohammadi A, Binder H, Kirste T, Teipel SJ, Alzheimer's Disease Neuroimaging Initiative. Comparison of different hypotheses regarding the spread of Alzheimer's disease using Markov random fields and multimodal imaging. Journal of Alzheimer's Disease. 2018Jan1;65(3):731-46
33. Silvestrelli G, Lanari A, Parnetti L, Tomassoni D, Amenta F. Treatment of Alzheimer's disease: from pharmacology to a better understanding of disease pathophysiology. Mechanisms of ageing and development. 2006Feb1;127(2):148-57
34. Anand R, Gill KD, Mahdi AA. Therapeutics of Alzheimer's disease: Past, present and future. Neuropharmacology. 2014Jan1;76:27-50
35. Kurz A, Perneczky R. Novel insights for the treatment of Alzheimer's disease. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2011Mar30;35(2):373-9
36. Dorsey EA, Constantinescu R, Thompson JP, Biglan KM, Holloway RG, Kieburtz K, Marshall FJ, Ravina BM, Schifitto G, Siderowf A, Tanner CM. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology. 2007Jan30;68(5):384-6
37. Kalia LV, Lang AE. Parkinson's disease. The Lancet. 2015Aug29;386(9996):896-912
38. Connolly BS, Lang AE. Pharmacological treatment of Parkinson disease: a review. Jama. 2014Apr23;311(16):1670-83
39. Pasquini J, Ceravolo R, Qamhawi Z, Lee JY, Deuschl G, Brooks DJ, Bonuccelli U, Pavese N. Progression of tremor in early stages of Parkinson's disease: a clinical and neuroimaging study. Brain. 2018Mar1;141(3):811-21
40. Factor SA, McDonald WM, Goldstein FC. The role of neurotransmitters in the development of Parkinson's disease-related psychosis. European Journal of Neurology. 2017Oct;24(10):1244-54
41. Schapira AH, Chaudhuri KR, Jenner P. Non-motor features of Parkinson disease. Nature Reviews Neuroscience. 2017Jul;18(7):435-50
42. Maillet A, Krack P, Lhommée E, Météreau E, Klinger H, Favre E, Le Bars D, Schmitt E, Bichon A, Pelissier P, Fraix V. The prominent role of serotonergic degeneration in apathy, anxiety and depression in de novo Parkinson's disease. Brain. 2016Sep1;139(9):2486-502
43. Morris R, Martini DN, Madhyastha T, Kelly VE, Grabowski TJ, Nutt J, Horak F. Overview of the cholinergic contribution to gait, balance and falls in Parkinson's disease. Parkinsonism & related disorders. 2019Jun1;63:20-30
44. Fox SH, Katzenschlager R, Lim SY, Barton B, De Bie RM, Seppi K, Coelho M, Sampaio C, Movement Disorder Society Evidence-Based Medicine Committee. International Parkinson and movement disorder society evidence-based medicine review: update on treatments for the motor symptoms of Parkinson's disease. Movement Disorders. 2018Aug;33(8):1248-66
45. Capildeo R, Haberman S, ROSE FC. The definition and classification of stroke: a new approach. QJM: An International Journal of Medicine. 1978Apr1;47(2):177-96
46. Portegies ML, Koudstaal PJ, Ikram MA. Cerebrovascular disease. Handbook of clinical neurology. 2016Jan1;138:239-61
47. Sim TM, Tarini D, Dheen ST, Bay BH, Srinivasan DK. Nanoparticle-based technology approaches to the management of neurological disorders. International Journal of Molecular Sciences. 2020Aug23;21(17):6070
48. Adams HP. Cancer and cerebrovascular disease. Current neurology and neuroscience reports. 2019Oct;19:1-10
49. Dobson R, Giovannoni G. Multiple sclerosis-a review. European journal of neurology. 2019Jan;26(1):27-40
50. Dolati S, Babaloo Z, Jadidi-Niaragh F, Ayromlou H, Sadreddini S, Yousefi M. Multiple sclerosis: Therapeutic applications of advancing drug delivery systems. Biomedicine & Pharmacotherapy. 2017Feb1;86:343-53
51. Ramagopalan SV, Dobson R, Meier UC, Giovannoni G. Multiple sclerosis: risk factors, prodromes, and potential causal pathways. The Lancet Neurology. 2010Jul1;9(7):727-39
52. McGinley MP, Goldschmidt CH, Rae-Grant AD. Diagnosis and treatment of multiple sclerosis: a review. Jama. 2021Feb23;325(8):765-79
53. Bruyn GW. Huntington's chorea: historical, clinical and laboratory synopsis. Handbook of Clinical Neurology. Elsevier Amsterdam. 1968 Volume 6: 298-378
54. Roos RA. Huntington's disease: a clinical review. Orphanet journal of rare diseases. 2010Dec;5:1-8
55. McColgan P, Tabrizi SJ. Huntington's disease: a clinical review. European journal of neurology. 2018Jan;25(1):24-34
56. MacDonald ME, Ambrose CM, Duyao MP, Myers RH, Lin C, Srinidhi L, Barnes G, Taylor SA, James M, Groot N, MacFarlane H. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. Cell. 1993Mar26;72(6):971-83
57. Ferguson MW, Kennedy CJ, Palpagama TH, Waldvogel HJ, Faull RL, Kwakowsky A. Current and possible future therapeutic options for Huntington's disease. Journal of Central Nervous System Disease. 2022Apr7;14:11795735221092517
58. Barbu E, Molnàr É, Tsibouklis J, Górecki DC. The potential for nanoparticle-based drug delivery to the brain: overcoming the blood-brain barrier. Expert opinion on drug delivery. 2009Jun1;6(6):553-65
59. Buzea C. et al. Nanomaterials and nanoparticles: Sources and toxicity. Biointerphases. 2007;2:MR17-71
60. Patel T, Zhou J, Piepmeier JM, Saltzman WM. Polymeric nanoparticles for drug delivery to the central nervous system. Advanced drug delivery reviews. 2012May15;64(7):701-5
61. Alam MS, Garg A, Pottoo FH, Saifullah MK, Tareq AI, Manzoor O, Mohsin M, Javed MN. Gum ghatti mediated, one pot green synthesis of optimized gold nanoparticles: investigation of process-variables impact using Box-Behnken based statistical design. International journal of biological macromolecules. 2017Nov1;104:758-67
62. Javed MN, Alam MS, Waziri A, Pottoo FH, Yadav AK, Hasnain MS, Almalki FA. QbD applications for the development of nanopharmaceutical products. InPharmaceutical quality by design. 2019 Jan 1 (pp. 229-253). Academic Press
63. Li SD, Huang L. Nanoparticles evading the reticuloendothelial system: role of the supported bilayer. Biochimica et Biophysica Acta (BBA)-Biomembranes. 2009Oct1;1788(10):2259-66
64. Klibanov AL, Maruyama K, Torchilin VP, Huang L. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS letters. 1990Jul30;268(1):235-7
65. Woodle MC, Collins LR, Sponsler E, Kossovsky N, Papahadjopoulos D, Martin FJ. Sterically stabilized liposomes. Reduction in electrophoretic mobility but not electrostatic surface potential. Biophysical journal. 1992Apr1;61(4):902-10
66. Ryvolova M, Chomoucka J, Drbohlavova J, Kopel P, Babula P, Hynek D, Adam V, Eckschlager T, Hubalek J, Stiborova M, Kaiser J. Modern micro and nanoparticle-based imaging techniques. Sensors. 2012Nov2;12(11):14792-820
67. Martins S, Sarmento B, Ferreira DC, Souto EB. Lipid-based colloidal carriers for peptide and protein delivery-liposomes versus lipid nanoparticles. International journal of nanomedicine. 2007Dec1;2(4):595-607
68. Lim SB, Banerjee A, Önyüksel H. Improvement of drug safety by the use of lipid-based nanocarriers. Journal of controlled release. 2012Oct10;163(1):34-45
69. Singh Dhakad R, Kumar Tekade R, Kumar Jain N. Cancer targeting potential of folate targeted nanocarrier under comparative influence of tretinoin and dexamethasone. Current Drug Delivery. 2013Aug1;10(4):477-91
70. Wissing SA, Kayser O, Müller RH. Solid lipid nanoparticles for parenteral drug delivery. Advanced drug delivery reviews. 2004May7;56(9):1257-72
71. Mara Mainardes R, Cristina Cocenza Urban M, Oliveira Cinto P, Vinicius Chaud M, Cesar Evangelista R, Palmira Daflon Gremiao M. Liposomes and micro/nanoparticles as colloidal carriers for nasal drug delivery. Current drug delivery. 2006Jul1;3(3):275-85
72. Nkanga CI, Bapolisi AM, Okafor NI, Krause RW. General perception of liposomes: formation, manufacturing and applications. Liposomes-advances and perspectives. 2019 Mar 26
73. Sahay G, Alakhova DY, Kabanov AV. Endocytosis of nanomedicines. Journal of controlled release. 2010Aug3;145(3):182-95
74. Hu FQ, Jiang SP, Du YZ, Yuan H, Ye YQ, Zeng S. Preparation and characteristics of monostearin nanostructured lipid carriers. International journal of pharmaceutics. 2006May11;314(1):83-9
75. Souto EB, Müller RH. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers Lipid Nanoparticles for Medicals and Pharmaceuticals. In Encyclopedia of Nanoscience and Nanotechnology. 2011 Jan 1 (Vol. 23, No. 328, pp. 313-328). American Scientific Publishers
76. Agrawal M, Saraf S, Saraf S, Dubey SK, Puri A, Patel RJ, Ravichandiran V, Murty US, Alexander A. Recent strategies and advances in the fabrication of nano lipid carriers and their application towards brain targeting. Journal of Controlled Release. 2020May10;321:372-415
77. Naseri N, Valizadeh H, Zakeri-Milani P. Solid lipid nanoparticles and nanostructured lipid carriers: structure, preparation and application. Advanced pharmaceutical bulletin. 2015Sep;5(3):305
78. Pottoo FH, Sharma S, Javed MN, Barkat MA, Harshita, Alam MS, Naim MJ, Alam O, Ansari MA, Barreto GE, Ashraf GM. Lipid-based nanoformulations in the treatment of neurological disorders. Drug Metabolism Reviews. 2020Jan2;52(1):185-204
79. Barot BS, Parejiya PB, Patel HK, Gohel MC, Shelat PK. Microemulsion-based gel of terbinafine for the treatment of onychomycosis: optimization of formulation using D-optimal design. Aaps Pharmscitech. 2012Mar;13:184-92
80. Kumara P, Mohanb C, Uma Shankara MK, Gulatia M. Physiochemical characterization and release rate studies of solid dispersions of Ketoconazole with Pluronic F127 and PVP K-30. Iran. J. Pharm. Res. 2011;10:685-94
81. Singh Y, Meher JG, Raval K, Khan FA, Chaurasia M, Jain NK, Chourasia MK. Nanoemulsion: Concepts, development and applications in drug delivery. Journal of controlled release. 2017Apr28;252:28-49
82. McClements DJ. Nanoemulsions versus microemulsions: terminology, differences, and similarities. Soft matter. 2012;8(6):1719-29
83. McClements DJ. Edible nanoemulsions: fabrication, properties, and functional performance. Soft matter. 2011;7(6):2297-316
84. Souto EB, Cano A, Martins-Gomes C, Coutinho TE, Zielińska A, Silva AM. Microemulsions and nanoemulsions in skin drug delivery. Bioengineering. 2022Apr5;9(4):158
85. Dhawan S, Kapil R, Singh B. Formulation development and systematic optimization of solid lipid nanoparticles of quercetin for improved brain delivery. Journal of Pharmacy and Pharmacology. 2011Mar;63(3):342-51
86. Pinheiro RG, Granja A, Loureiro JA, Pereira MC, Pinheiro M, Neves AR, Reis S. Quercetin lipid nanoparticles functionalized with transferrin for Alzheimer's disease. European Journal of Pharmaceutical Sciences. 2020May30;148:105314
87. Pinheiro RG, Granja A, Loureiro JA, Pereira MC, Pinheiro M, Neves AR, Reis S. RVG29-functionalized lipid nanoparticles for quercetin brain delivery and Alzheimer's disease. Pharmaceutical Research. 2020Jul;37:1-2
88. Dara T, Vatanara A, Sharifzadeh M, Khani S, Vakilinezhad MA, Vakhshiteh F, Meybodi MN, Malvajerd SS, Hassani S, Mosaddegh MH. Improvement of memory deficits in the rat model of Alzheimer's disease by erythropoietin-loaded solid lipid nanoparticles. Neurobiology of learning and memory. 2019Dec1;166:107082
89. Saffari PM, Alijanpour S, Takzaree N, Sahebgharani M, Etemad-Moghadam S, Noorbakhsh F, Partoazar A. Metformin loaded phosphatidylserine nanoliposomes improve memory deficit and reduce neuroinflammation in streptozotocin-induced Alzheimer's disease model. Life Sciences. 2020Aug15;255:117861
90. Jiang Y, Liu C, Zhai W, Zhuang N, Han T, Ding Z. The optimization design of lactoferrin loaded HupA nanoemulsion for targeted drug transport via intranasal route. International journal of nanomedicine. 2019Nov;27:9217-34
91. Dudhipala N, Gorre T. Neuroprotective effect of ropinirole lipid nanoparticles enriched hydrogel for parkinson's disease: In vitro, ex vivo, pharmacokinetic and pharmacodynamic evaluation. Pharmaceutics. 2020May13;12(5):448
92. Uppuluri CT, Ravi PR, Dalvi AV. Design, optimization and pharmacokinetic evaluation of Piribedil loaded solid lipid nanoparticles dispersed in nasal in situ gelling system for effective management of Parkinson's disease. International Journal of Pharmaceutics. 2021Sep5;606:120881
93. Joy D, Jose J, Bibi S, Bandiwadekar A, Gopan G, Mariana Gonçalves Lima C, Bin Emran T, A Alhumaydhi F, Ashtekar H, Adam Conte-Junior C. Development of microneedle patch loaded with bacopa monnieri solid lipid nanoparticles for the effective management of Parkinson's disease. Bioinorganic Chemistry and Applications. 2022 Aug 10. 2022
94. Mani M, Balasubramanian S, Manikandan KR, Kulandaivel B. Neuroprotective potential of Naringenin-loaded solid-lipid nanoparticles against rotenone-induced Parkinson's disease model. Journal of Applied Pharmaceutical Science. 2021Feb5;11(2):019-28
95. Rahman M, Kumar V. Fabrication of solid lipid nanoparticles containing vitexin protects dopaminergic neurons against 6-hydroxydopamine induced Parkinson's disease model via altered the genetic backgrounds. Journal of the Neurological Sciences. 2019Oct15;405:248
96. Tsai MJ, Wu PC, Huang YB, Chang JS, Lin CL, Tsai YH, Fang JY. Baicalein loaded in tocol nanostructured lipid carriers (tocol NLCs) for enhanced stability and brain targeting. International journal of pharmaceutics. 2012Feb28;423(2):461-70
97. Hassanzadeh P, Arbabi E, Atyabi F, Dinarvand R. Ferulic acid-loaded nanostructured lipid carriers: A promising nanoformulation against the ischemic neural injuries. Life sciences. 2018Jan15;193:64-76
98. Gao Y, Gu W, Chen L, Xu Z, Li Y. The role of daidzein-loaded sterically stabilized solid lipid nanoparticles in therapy for cardio-cerebrovascular diseases. Biomaterials. 2008Oct1;29(30):4129-36
99. Al-Ahmady ZS, Dickie BR, Aldred I, Jasim DA, Barrington J, Haley M, Lemarchand E, Coutts G, Kaur S, Bates J, Curran S. Selective brain entry of lipid nanoparticles in haemorrhagic stroke is linked to biphasic blood-brain barrier disruption. Theranostics. 2022;12(10):4477
100. Wu S, Liao D, Li X, Liu Z, Zhang L, Mo FM, Hu S, Xia J, Yang X. Endogenous oleoylethanolamide crystals loaded lipid nanoparticles with enhanced hydrophobic drug loading capacity for efficient stroke therapy. International Journal of Nanomedicine. 2021Dec;21:8103-15
101. Sabry SA, Abd El Razek AM, Nabil M, Khedr SM, El-Nahas HM, Eissa NG. Brain-targeted delivery of Valsartan using solid lipid nanoparticles labeled with Rhodamine B; a promising technique for mitigating the negative effects of stroke. Drug Delivery. 2023Dec31;30(1):2179127
102. Ojha S, Kumar B. Preparation and statistical modeling of solid lipid nanoparticles of dimethyl fumarate for better management of multiple sclerosis. Advanced pharmaceutical bulletin. 2018Jun;8(2):225
103. Kumar P, Sharma G, Gupta V, Kaur R, Thakur K, Malik R, Kumar A, Kaushal N, Katare OP, Raza K. Oral delivery of methylthioadenosine to the brain employing solid lipid nanoparticles: pharmacokinetic, behavioral, and histopathological evidences. AAPS PharmSciTech. 2019Feb;20:1-7
104. Gandomi N, Varshochian R, Atyabi F, Ghahremani MH, Sharifzadeh M, Amini M, Dinarvand R. Solid lipid nanoparticles surface modified with anti-Contactin-2 or anti-Neurofascin for brain-targeted delivery of medicines. Pharmaceutical Development and Technology. 2017Apr3;22(3):426-35
105. Sandhir R, Yadav A, Mehrotra A, Sunkaria A, Singh A, Sharma S. Curcumin nanoparticles attenuate neurochemical and neurobehavioral deficits in experimental model of Huntington's disease. Neuromolecular medicine. 2014Mar;16:106-18
106. Bhatt R, Singh D, Prakash A, Mishra N. Development, characterization and nasal delivery of rosmarinic acid-loaded solid lipid nanoparticles for the effective management of Huntington's disease. Drug delivery. 2015Oct3;22(7):931-9
107. Ramachandran S, Thangarajan S. A novel therapeutic application of solid lipid nanoparticles encapsulated thymoquinone (TQ-SLNs) on 3-nitroproponic acid induced Huntington's disease-like symptoms in wistar rats. Chemico-Biological Interactions. 2016Aug25;256:25-36
108. Verma S, Singla M, Gupta S, Porwal O, Nasser Binjawhar D, Sayed AA, MIttal DP, El-Demerdash FM, Algahtani M, SINGH SK, Dua K. Theoretical design for covering Engletin with functionalized nanostructure-lipid carriers as neuroprotective agents against Huntington's disease via the nasal-brain route. Frontiers in Pharmacology.;14:1218625.
109. Yusuf M, Khan M, Khan RA, Ahmed B. Preparation, characterization, in vivo and biochemical evaluation of brain targeted Piperine solid lipid nanoparticles in an experimentally induced Alzheimer's disease model. Journal of drug targeting. 2013Apr1;21(3):300-11
110. Orlando A, Re F, Sesana S, Rivolta I, Panariti A, Brambilla D, Nicolas J, Couvreur P, Andrieux K, Masserini M, Cazzaniga E. Effect of nanoparticles binding β-amyloid peptide on nitric oxide production by cultured endothelial cells and macrophages. International Journal of Nanomedicine. 2013Apr;15:1335-47
111. Kakkar V, Muppu SK, Chopra K, Kaur IP. Curcumin loaded solid lipid nanoparticles: an efficient formulation approach for cerebral ischemic reperfusion injury in rats. European Journal of Pharmaceutics and Biopharmaceutics. 2013Nov1;85(3):339-45
112. Lu YM, Huang JY, Wang H, Lou XF, Liao MH, Hong LJ, Tao RR, Ahmed MM, Shan CL, Wang XL, Fukunaga K. Targeted therapy of brain ischaemia using Fas ligand antibody conjugated PEG-lipid nanoparticles. Biomaterials. 2014Jan1;35(1):530-7
113. Parikh A, Kathawala K, Tan CC, Garg S, Zhou XF. Lipid-based nanosystem of edaravone: development, optimization, characterization and in vitro/in vivo evaluation. Drug delivery. 2017Jan1;24(1):962-78
114. Shah B, Khunt D, Bhatt H, Misra M, Padh H. Application of quality by design approach for intranasal delivery of rivastigmine loaded solid lipid nanoparticles: effect on formulation and characterization parameters. European journal of pharmaceutical sciences. 2015Oct12;78:54-66
115. Muntimadugu E, Dhommati R, Jain A, Challa VG, Shaheen M, Khan W. Intranasal delivery of nanoparticle encapsulated tarenflurbil: a potential brain targeting strategy for Alzheimer's disease. European journal of pharmaceutical sciences. 2016Sep20;92:224-34
116. Zhao YZ, Li X, Lu CT, Lin M, Chen LJ, Xiang Q, Zhang M, Jin RR, Jiang X, Shen XT, Li XK. Gelatin nanostructured lipid carriers-mediated intranasal delivery of basic fibroblast growth factor enhances functional recovery in hemiparkinsonian rats. Nanomedicine: Nanotechnology, Biology and Medicine. 2014May1;10(4):755-64
117. Tsai MJ, Huang YB, Wu PC, Fu YS, Kao YR, Fang JY, Tsai YH. Oral apomorphine delivery from solid lipid nanoparticles with different monostearate emulsifiers: pharmacokinetic and behavioral evaluations. Journal of pharmaceutical sciences. 2011Feb1;100(2):547-57
118. Kundu P, Das M, Tripathy K, Sahoo SK. Delivery of dual drug loaded lipid based nanoparticles across the blood-brain barrier impart enhanced neuroprotection in a rotenone induced mouse model of Parkinson's disease. ACS chemical neuroscience. 2016Dec21;7(12):1658-70
119. Tredicine M, Ria F, Poerio N, Lucchini M, Bianco A, De Santis F, Valentini M, De Arcangelis V, Rende M, Stabile AM, Pistilli A. Liposome-based nanoparticles impact on regulatory and effector phenotypes of macrophages and T cells in multiple Sclerosis patients. Biomaterials. 2023Jan1;292:121930
120. Binyamin O, Larush L, Frid K, Keller G, Friedman-Levi Y, Ovadia H, Abramsky O, Magdassi S, Gabizon R. Treatment of a multiple sclerosis animal model by a novel nanodrop formulation of a natural antioxidant. International Journal of Nanomedicine. 2015Nov;20:7165-74
121. Hirunagi T, Sahashi K, Tachikawa K, Leu AI, Nguyen M, Mukthavaram R, Karmali PP, Chivukula P, Tohnai G, Iida M, Onodera K. Selective suppression of polyglutamine-expanded protein by lipid nanoparticle-delivered siRNA targeting CAG expansions in the mouse CNS. Molecular Therapy-Nucleic Acids. 2021Jun4;24:1-10
Author contact
![]() Corresponding author: Md. Rajdoula Rafe. Email: mrrafe2-ccityu.edu.hk & rajdoulajnu.ac.bd.
Corresponding author: Md. Rajdoula Rafe. Email: mrrafe2-ccityu.edu.hk & rajdoulajnu.ac.bd.
Received 2023-8-5
Accepted 2023-9-5
Published 2024-1-1
