ISSN: 2206-7418Nanotheranostics
Nanotheranostics 2017; 1(2):141-153. doi:10.7150/ntno.18897 This issue Cite
Research Paper
Satellite-like Gold Nanocomposites for Targeted Mass Spectrometry Imaging of Tumor Tissues
1. Department of Chemistry, National Taiwan University, Taipei 10617, Taiwan;
2. Department of Chemistry, Université de Montréal, Montréal, Québec H3C 3J7, Canada;
3. Department of Bioscience and Biotechnology, National Taiwan Ocean University, Keelung 20224, Taiwan;
4. Department of Chemistry, Chung Yuan Christian University, Taoyuan City 32023, Taiwan;
5. Center of Excellence for the Oceans, National Taiwan Ocean University, Keelung 20224, Taiwan;
6. School of Pharmacy, College of Pharmacy, Kaohsiung Medical University, Kaohsiung 80708, Taiwan.
Abstract
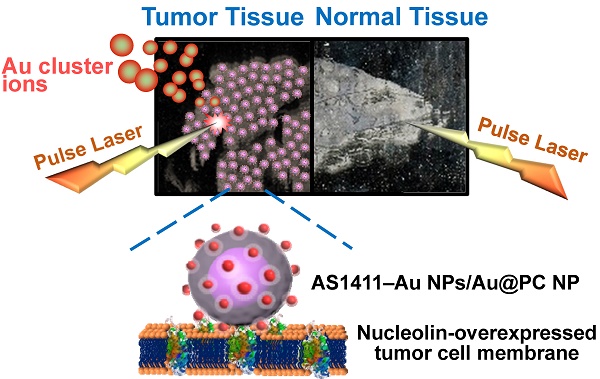
We have developed a simple, rapid, high-throughput cancer diagnosis system using functional nanoparticles (NPs) consisting of poly(catechin) capped-gold NPs (Au@PC NPs) and smaller nucleolin-binding aptamer (AS1411) conjugated gold NPs (AS1411-Au NPs). The AS1411-Au NPs/Au@PC NP is used as a targeting agent in laser desorption/ionization mass spectrometry (LDI-MS)-based tumor tissue imaging. Self-assembled core-shell Au@PC NPs are synthesized by a simple reaction of tetrachloroaurate(III) with catechin. Au@PC NPs with a well-defined and dense poly(catechin) shell (~40-60 nm) on the surface of each Au core (~60-80 nm) are obtained through careful control of the ratio of catechin to gold ions, as well as the pH of the reaction solution. Furthermore, we have shown that AS1411-conjugated Au NPs (13-nm) self-assembled on Au@PC NP can from a satellite-like gold nanocomposite. The high density of AS1411-Au NPs on the surface of Au@PC NP enhances multivalent binding with nucleolin molecules on tumor cell membranes. We have employed LDI-MS to detect AS1411-Au NPs/Au@PC NPs labeled nucleolin-overexpressing MCF-7 breast cancer cells through the monitoring of Au cluster ions ([Aun]+; 1 ≤ n ≤ 3). The ultrahigh signal amplification from Au NPs through the formation of a huge number of [Aun]+ ions results in a sensing platform with a limit of detection of 100 MCF-7 cells mL-1. Further, we have applied the satellite-like AS1411-Au NPs/Au@PC NP nanocomposite as a labeling agent for tumor tissue imaging by LDI-MS. Our nanocomposite-assisted LDI-MS imaging platform can be extended for simultaneous analysis of different tumor markers on cell membranes when using different ligand-modified metal nanoparticles.
Keywords: self-assembly, aptamers, gold nanocomposites, laser desorption/ionization mass spectrometry, tissue imaging

 Global reach, higher impact
Global reach, higher impact