ISSN: 2206-7418
Nanotheranostics 2017; 1(3):232-243. doi:10.7150/ntno.19952 This issue Cite
Research Paper
Accumulation of 111In-Labelled EGF-Au-PEG Nanoparticles in EGFR-Positive Tumours is Enhanced by Coadministration of Targeting Ligand
1. CR-UK/MRC Oxford Institute for Radiation Oncology, Department of Oncology, University of Oxford, Oxford, OX3 7DQ, UK;
2. Sir William Dunn School of Pathology, University of Oxford, Oxford, OX1 3RE, UK.
Abstract
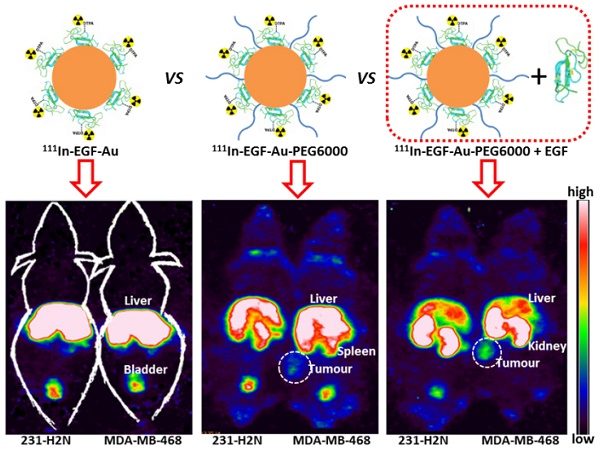
The successful use of targeted radionuclide therapy in the treatment of solid tumours may be limited by radioresistance, which necessitates delivery of a high dose of radioactivity. Nanoparticle (NP)-based delivery systems possess a large surface area for attachment of radioisotopes and so offer a solution to this challenge. However, tumour uptake may be limited by rapid hepatic clearance of NP via the mononuclear phagocyte system. Liver uptake is further compounded when epidermal growth factor (EGF) is used as a targeting ligand, as EGF-tagged NP bind the EGF receptor (EGFR), which is expressed to a moderate extent by hepatocytes. This report describes an indium-111 (111In)-labelled PEGylated EGF-tagged gold (Au) NP (111In-EGF-Au-PEG) and an effective strategy of coadministration of targeting ligand to address these issues. Direct attachment of EGF to the surface of Au NP did not compromise surface coating with long-chain PEG. In vitro experiments showed that 111In-EGF-Au-PEG targets EGFR-positive cancer cells (MDA-MB-468): >11% of radioactivity was internalised after incubation for 4 h. In in vivo studies accumulation of NP was observed in MDA-MB-468 xenografts and tumour uptake was enhanced by the coadministration of 15 µg of the unlabelled targeting ligand, EGF, to block hepatic EGFR. Uptake was 3.9% versus 2.8% injected dose/g (%ID/g) of tumour tissue with and without unlabelled EGF, respectively. Coadministration of EGF reduced liver uptake by 25.95% to 7.56 %ID/g. This suggests that the coadministration of unlabelled targeting ligand with radiolabelled PEGylated NP offers a promising strategy for targeting EGFR-positive cancer and for minimising liver uptake.
Keywords: gold nanoparticle, EGF, radiolabelling, 111In, cancer targeting, coadministration.

 Global reach, higher impact
Global reach, higher impact