ISSN: 2206-7418
Nanotheranostics 2017; 1(3):326-337. doi:10.7150/ntno.20233 This issue Cite
Research Paper
TRAIL-functionalized gold nanoparticles selectively trigger apoptosis in polarized macrophages
1. Institute of Polymer Science and Engineering, National Taiwan University, Taipei, Taiwan, R.O.C.
2. Institute of Cellular and System Medicine, National Health Research Institutes, Miaoli County, Taiwan, R.O.C.
Abstract
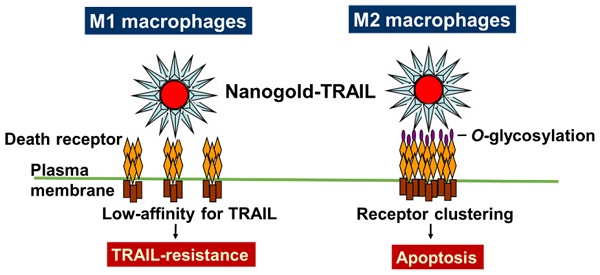
Tumor-associated macrophages (TAMs) have the same immunosuppressive effects as M2 macrophages in tumor progression and are correlated with poor-patient prognosis and survival in non-small cell lung cancer (NSCLC). Therefore, TAMs are the potential targets for cancer therapy. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is a member of tumor necrosis factor superfamily and selectively induces cancer cell apoptosis, but not in most normal cells. Nanoparticles coated with multiple ligands can act as multivalent ligands that may actively crosslink cell surface receptors to affect downstream signals. Here, we explored nanogolds coated with TRAIL protein (nanogold-TRAIL complexes) as a potential anti-M2 macrophage drug. The structure of nanogold-TRAIL complexes comprised nanogold (3, 13, or 30 nm) as the core to crosslink multiple TRAIL for exhibition of multivalent property. Nanogold-TRAIL complexes selectively increased the cytotoxicity of TRAIL (30-fold increase in IC50) via changing O-glycosylation levels in M2-polarized macrophages. By testing the TRAIL complex efficacy on nanogold with different sizes and origins as well as on superparamagnetic iron oxide nanoparticles, we further demonstrated that the enhanced cytotoxicity by nanoparticles was dependent on size and surface properties of the nanoparticles. Meanwhile, the nanogold-TRAIL complexes remained nontoxic to M1 macrophages or normal cells. Nanogold-TRAIL complexes thus provide a novel and promising strategy for the improvement of TRAIL-based therapy.
Keywords: tumor necrosis factor-related apoptosis-inducing ligand, tumor-associated macrophages, nanogold, apoptosis, O-glycosylation.

 Global reach, higher impact
Global reach, higher impact