ISSN: 2206-7418
Nanotheranostics 2020; 4(3):156-172. doi:10.7150/ntno.44703 This issue Cite
Research Paper
Supramolecular Polysaccharide Nanotheranostics that Inhibit Cancer Cells Growth and Monitor Targeted Therapy Response
1. Department of Chemical Engineering, University of Massachusetts, Amherst, MA, USA
2. Depatment of Biomedical Engineering, University of Massachusetts, Amherst, MA, USA
3. Department of Veterinary and Animal Science, University of Massachusetts, Amherst, MA, USA
4. Center for Bioactive Delivery, Institute for Applied Life Sciences, University of Massachusetts, Amherst, MA, USA
Abstract
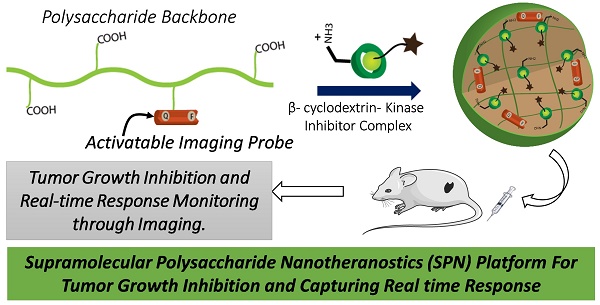
Targeted anticancer therapies directed against specific molecular drivers of tumors are emerging as effective treatment strategies, however, monitoring their response is still challenging. Current clinical imaging techniques that measure either morphological or metabolic changes in the tumor are not always indicative of clinical outcome due to delayed or variable responses. Here, dual-stage polysaccharide-based supramolecular nanotheranostics (SPN) were designed that enable co-delivery of kinase inhibitor and an activatable imaging probe.
Methods: The SPNs were prepared by supramolecular assembly of two components, polysaccharide construct conjugated with kinase inhibitor-function activatable probe and kinase inhibitor- β-cyclodextrin conjugate. Physiochemical characterization of SPNs including size, stability, zeta potential and pH-responsiveness of the assembly was performed. The efficacy of SPNs in inducing cancer cell death by inhibition of kinase signaling and imaging the response was evaluated in murine BRAFV600E melanoma (D4M) and triple-negative breast cancer (4T1) cell lines. Finally, the in vivo efficacy was investigated in D4M melanoma tumor model.
Results: The polysaccharide-constructs along with kinase inhibitor- β-cyclodextrin conjugates self-assemble to produce SPNs of around 200 nm in diameter and were stable for over a week under physiologically relevant conditions. The SPNs exhibited enhanced cytotoxic effect and significant inhibition of kinase signaling as compared to the free inhibitor. In vitro imaging studies confirmed their enzyme-activatable therapy response tracking abilities both in cancer cells and tumor spheroids. Furthermore, SPN treated mice exhibited better tumor growth inhibition as compared to the control groups and therapy response could be imaged at both early (24-48h) and later time points.
Conclusion: These findings demonstrate that the supramolecular polysaccharide nanotheranostics can not only inhibit kinase signaling pathway in aggressive tumor, but also monitor targeted therapy response early.
Keywords: Polysaccharide, Nanotheranostics, Supramolecular Chemistry, Kinase inhibitor Delivery, Cancer

 Global reach, higher impact
Global reach, higher impact