ISSN: 2206-7418
Nanotheranostics 2021; 5(4):472-487. doi:10.7150/ntno.62364 This issue Cite
Research Paper
Photoactive Silver Nanoagents for Backgroundless Monitoring and Precision Killing of Multidrug-Resistant Bacteria
1. State Key Laboratory of Medicinal Chemical Biology, Research Center for Analytical Sciences, Tianjin Key Laboratory of Biosensing and Molecular Recognition, College of Chemistry, Nankai University, Tianjin 300071, China.
2. Department of Intensive Care Unit, Key Laboratory for Critical Care Medicine of the Ministry of Health, Emergency Medicine Research Institute, Tianjin First Center Hospital, School of Medicine, Nankai University, Tianjin 300071, China.
3. Key Laboratory of Functional Polymer Materials of Ministry of Education, Institute of Polymer Chemistry, College of Chemistry, Nankai University, Tianjin 300071, China.
*Present address: Key Laboratory of Biomedical Polymers of Ministry of Education, Department of Chemistry, Wuhan University, Wuhan 430072, China.
Abstract
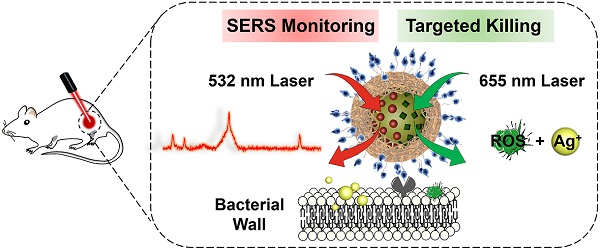
Purpose: The growing prevalence of multidrug-resistant (MDR) bacteria makes it clinically urgent to develop an agent able to detect and treat infections simultaneously. Silver has served as a broad-spectrum antimicrobial since ancient times but suffers from major challenges such as moderate antimicrobial activity, nonspecific toxicity, and difficulty to be visualized in situ. Here, we propose a new photoactive silver nanoagent that relies on a photosensitizer-triggered cascade reaction to liberate Ag+ on bacterial surfaces exclusively, allowing the precise killing of MDR bacteria. Additionally, the AgNP core acts as a backgroundless surface-enhanced Raman scattering (SERS) substrate for imaging the distribution of the nanoagents on bacterial surfaces and monitoring their metabolic dynamics in the infection sites.
Methods: In this strategy, the photoactive antibacterial AgNP was decorated with photosensitizers (Chlorin e6, Ce6) and Raman reporter (4-Mercaptobenzonitrile, 4-MB) to provide new opportunities for clinically monitoring and fighting MDR bacterial infections. Upon 655 nm laser activation, the Ce6 molecules produce ROS efficiently, triggering the rapid release of Ag+ from the AgNP core to kill bacteria. Poly[4-O-(α-D-glucopyranosyl)-D-glucopyranose] (GP) was introduced as bacteria-specific targeting ligands. SERS spectra of the prepared GP-Ce6/MB-AgNPs were recorded after injecting for 0.5, 4, 8, 12, 24, and 48 h to track the dynamic metabolism of the nanoagents and thus guiding the antibacterial therapy.
Results: This new antimicrobial strategy exerts a dramatically enhanced antibacterial activity. The in vitro antibacterial efficiencies of this non-antibiotic technique were up to 99.6% against Methicillin-resistant Staphylococcus aureus (MRSA) and 98.8% against Escherichia coli (EC), while the in vivo antibacterial efficiencies for MRSA- and Carbapenem-resistant Pseudomonas aeruginosa (CRPA)-infected mice models were 96.8% and 93.6%, respectively. Besides, backgroundless SERS signal intensity of the wound declined to the level of normal tissue until 24 h, indicating that the nanoagents had been completely metabolized from the infected area.
Conclusion: Given the backgroundless monitoring ability, high antibacterial efficacy, and low toxicity, the photoactive cascading agents would hold great potential for MDR-bacterial detection and elimination in diverse clinical settings.
Keywords: multidrug-resistant bacteria, silver nanoagent, backgroundless Raman technology, targeted killing, bacterial imaging

 Global reach, higher impact
Global reach, higher impact