ISSN: 2206-7418
Nanotheranostics 2022; 6(3):325-336. doi:10.7150/ntno.69259 This issue Cite
Research Paper
EK-16A liposomes enhance HIV replication in ACH2 or J-Lat 10.6 cell engrafted NSG mice
1. State Key Laboratory of Genetic Engineering and Engineering Research Center of Gene Technology, Ministry of Education, Institute of Genetics, School of Life Sciences, Fudan University, Shanghai, China.
2. Department of Infectious Disease, Key Laboratory of Medical Molecular Virology of Ministry of Education/Health, School of Basic Medical Sciences and Shanghai Public Health Clinical Center, Fudan University, Shanghai, China.
Received 2021-11-23; Accepted 2022-2-25; Published 2022-3-21
Abstract
Background: Numbers of HIV latency reversal agents (LRAs) have been tested in clinical trials, but with limited effect. EK-16A is an ingenol derivative that isolated from Euphorbia kansui. Our prior studies have suggested that it could reactivate latent HIV and meanwhile inhibit HIV infection in vitro. Here, we further advanced the research in vivo.
Methods: In vitro, the activity of EK-16A liposomes was measured in HIV latently infected cells. In serum pharmacology test, BALB/c mice were orally administered with EK-16A liposomes, serum was separated and co-cultured with cells, HIV reactivation was measured. In vivo, NSG mice were transplanted with human cells for 3 weeks and then administered with EK-16A liposomes for 3 days. In ACH2 cell engrafted NSG mice, P24 in plasma and cell-associated HIV RNA in tissues was measured. In J-Lat 10.6 cell engrafted NSG mice, GFP expression of J-Lat 10.6 cells in diverse tissues was measured. Hematoxylin and eosin (HE) staining was carried out for histopathological examination in both mice.
Results: EK-16A liposomes can reactivate latent HIV in ACH2 and J-Lat 10.6 cells. Serum pharmacological test showed that EK-16A retained activity after oral administration. Importantly, in ACH2 cell engrafted NSG mice, EK-16A liposomes increased the secretion of P24 in plasma and the expression of cell-associated HIV RNA in tissues. In J-Lat 10.6 cell engrafted NSG mice, EK-16A liposomes increased the GFP expression of J-Lat 10.6 cells in diverse tissues, including the bone marrow, spleen, liver, lung and peripheral blood. Furthermore, there was no obvious histopathological change associated with the use of EK-16A liposomes in both mice.
Conclusions: Our results confirmed the enhancing HIV replication activity and preliminary security of EK-16A in human cell engrafted NSG mice, laying the foundation for research in clinical trials.
Keywords: HIV, LRAs, Euphorbia kansui, EK-16A, NSG mouse model
Introduction
Antiretroviral therapy (ART) effectively suppresses viral replication and partially restores immune functions in HIV infected individuals [1-3]. However, ART cannot eliminate transcriptionally silent proviruses. A small pool of resting cells harbors latent replication competent HIV, and this latent reservoir remains untouched by current ART [4-6]. It represents a key challenge in the eradication of HIV [7, 8]. One potential strategy for eliminating the latently infected cells is termed as “shock and kill” in which LRAs are used to shock the latent virus out of the resting cells to allow host immune system, with the assistance of immunomodulatory therapies, to kill the virus ex pressing cells. This “shock and kill” procedure would typically be performed in those receiving ART to prevent additional rounds of viral replication [9].
A variety of compounds are under investigation as candidate LRAs for the shock step. Epigenetic LRAs targeted the chromatin restrictions that maintain HIV latency. These LRAs fall into three groups: histone deacetylase inhibitors [10-13], histone methyltransferase inhibitors [14] and bromo- and extra-terminal domain inhibitors [15-17]. Signal Agonist LRAs stimulate immune signaling pathways in T cells, leading to transcriptional modifications and NF-κB activation. These LRAs include PKC agonists [18], cytokines [19-21], Toll-like receptor agonists [22-24], mimetics of the second mitochondrial-derived activator of caspases [25], and immune checkpoint inhibitors [26, 27]. Clinical trials using SAHA [10, 28-31], romidepsin [13, 32], panobinostat [11], bryostatin-1 [33], disulfiram [34] and so on showed some promise, but failed to result in significant reduction in the frequency of infected cells [19]. It is urgent to develop more potent and safer LRAs.
Euphorbia kansui has been prescribed for thousands of years in traditional Chinese medicine. We previously reported that effective fractions from the dichloromethane extracts of the root of Euphorbia kansui can reactivate latent HIV replication in different latent cells (The 24th China science technology Forum-High level Forum on HIV cure, December 16-17, 2012, Beijing), and obtained the Chinese patent application approval and authorization (CN102727563B, CN106928063A). Recently, Euphorbia kansui has received increasing scientific attention for HIV latency reversal [35, 36], other researchers and we have launched clinical trials successively (clinicaltrials.gov, Identifier NCT02531295, NCT04503928). EK-16A is an ingenol derivative that extracted from Euphorbia kansui [37]. Our prior studies have suggested that it could reactivate latent HIV by inducing both NF-κB and P-TEFb signaling pathways [38] and meanwhile inhibit HIV infection by down-regulating the expression of cell surface HIV co-receptors CCR5 and CXCR4 [39] in vitro. In the current study, EK-16A was prepared into liposomes. The activity of EK-16A liposomes on enhancing HIV replication was evaluated in vitro and importantly in ACH2 and J-Lat 10.6 cell engrafted NSG mice in vivo.
Methods
Preparation of reagents
EK-16A is a chemical compound that isolated from Euphorbia kansui and the structure is as described in the previous study [38]. In the current study, it was prepared as liposomes. Briefly, lecithin and cholesterol (Kewpie Corporation) were dissolved in dichloromethane, into which EK-16A solution (1 mg/mL) was added. The mixture formed a w/o emulsion upon sonication, which was subsequently evaporated under reduced pressure with a rotating speed of 50 rpm at 40 ℃ to remove the organic solvents. After that, PBS (pH 7.4) was added to hydrate the lipids until a homogeneous dispersion was formed. Finally, this dispersion was extruded though a high-pressure homogenizer to obtain liposomes [40]. Vehicle control was prepared by the same procedure but EK-16A was not included in formulation. SAHA (Selleckchem) was suspended in sterile water plus 0.5% methylcellulose and 0.1% Tween [41].
Cell culture
ACH2 cells are a CEM T-cell clone containing one integrated proviral copy of latent HIV-1 LAV [42, 43]. J-Lat 10.6 cells are a Jurkat T-cell clone containing a full-length integrated HIV genome that expresses GFP upon activation [44]. Both cell lines were obtained from NIH AIDS Reagent Program. They were cultured in RPMI1640 medium with 10% fetal bovine serum (FBS) and 1% Pen/strep in a 37 °C incubator containing 5% CO2.
Ethics statement
All animal studies were reviewed and approved by Animal Ethic Committee of Fudan University.
Measurement of HIV reactivation in vitro
ACH2 cells or J-Lat 10.6 cells were added at equal amounts for a total of 5×105 cells per well to 24-well plates containing compound titrations. Dose-response testing was performed on EK-16A liposomes dissolved in PBS to give final assay concentrations ranging from 100 ng/mL to 0.16 ng/mL in 500 μL of medium. Cells and compound were incubated for 48 h. HIV reactivation in ACH2 cells was measured by the secretion of P24 antigen in supernatant using HIV Gag P24 ELISA kit (R&D Systems). HIV reactivation in J-Lat 10.6 cells was measured by the expression of GFP using flow cytometry (Beckman Coulter) [45]. Data were analyzed using FlowJo software (V10).
Serum pharmacological test
BALB/c mice aged 6 weeks were purchased from Shanghai SLAC Laboratory Animal Co., Ltd and randomized for assignment to either experimental or control groups. EK-16A liposomes were orally administered once daily at 0, 0.5 or 1mg/kg body weight for 3 days. Before drug administration, and after the last administration for 1 h, 2 h and 3 h, blood was collected from the retro-orbital venous sinus (r.o.). Serum was acquired by centrifugation the sample at 800 g for 10 min and then was calefied in 56 °C water for 30 min. Finally, 450 μL cells (5 × 105) were co-cultured with 50 μL serum for 48 h and HIV induction was measured as in vitro [46-50].
Cytotoxicity assay
This assay was performed as the protocol of the Cell Counting Kit-8 (CCK-8) (Dojindo Molecular Technologies). Briefly, cells were treated with EK-16A liposomes or serum from mice for 48 h. Then CCK-8 solution was added to each well. After 4 h of incubation at 37 °C, the absorbance at 450 nm was measured using a microplate reader (BioTec) [38].
Cell surface marker staining
Cells were washed once with PBS, resuspended in 100 µL PBS, stained with human CD147-APC (BioLegend), CD29-APC (BioLegend), HLA-ABC-APC (BD), CD3-PE (BD), CD4-PE (Miltenyi) or TCR α β-FITC (BD) antibodies, respectively, and were incubated for 30 min. Cells were then washed with 1 mL of PBS, resuspended in 500 µL PBS, and measured by flow cytometry [51].
Establishment and analysis of cell engrafted NSG mice
NSG mice (NOD-PrkdcscidIl2rgem1/Smoc, NM-NSG-001) [52-54] aged 6 weeks were purchased from Shanghai Model Organisms. NSG mice were transplanted with 1 × 107 ACH2 cells or J-Lat 10.6 cells in a volume of 200 µL PBS by tail vein injection. Cell engrafted mice as well as control mice were sacrificed by cervical dislocation at the indicated time. Bone marrow, spleen, liver, lung, and peripheral blood were harvested. Single cell suspensions from these tissues were prepared as described previously [51, 55]. For the measurement of engraftment, cells were stained with human CD147-APC and measured by flow cytometry [51].
Treatment of cell engrafted NSG mice
NSG mice were transplanted with ACH2 cells or J-Lat 10.6 cells. Three weeks post-transplantation, they were randomized for assignment to either experimental or control groups. Cell engrafted mice respectively received 3 times of EK-16A liposomes (1 mg/kg), SAHA (60 mg/kg) or vehicle control orally. They were euthanized 24 h after the last dose.
Measurement of HIV reactivation in vivo
In ACH2 cell engrafted mice, HIV induction was analyzed by monitoring the change of plasma P24 antigen before and after treatment. The level of P24 antigen in plasma was determined using HIV Gag P24 ELISA kit according to the manufacturer's protocol. HIV induction was also analyzed by comparing tissue HIV RNA of LRA treated mice versus the vehicle control [25]. Single cell suspensions from tissues were prepared. RNA was extracted using ZR-96 Viral RNA Kit (ZYMO Research). cDNA was synthesized using BeyoRT III cDNA kit (Beyotime) and analysed using QuantiFast SYBR Green PCR Kit (Qiagen) on a Roche LightCycler 480 II machine. HIV gag expression was normalized to GAPDH and the comparative threshold cycle (Ct) method (ΔΔCt) was used for relative quantification of gene expression. Results were presented as fold induction relative to vehicle control. HIV viral RNA levels in cells isolated from the bone marrow, spleen, liver, lung and peripheral blood of control or LRA-treated mice (cells pooled from n = 5 mice per group for each tissue) were analyzed in triplicate. The primer sequences of HIV gag detection were: 5′- ACATCAAGCAGCCATGCAAAT-3′, 5′-TCTGGCCTGGTGCAATAGG-3′. The primer sequences of GAPDH detection were: 5′-TCAAGTGGGGCGATGCTGGC-3′, 5′-TGGGGGCATCAGCAGAGGGG-3′ [56].
In J-Lat 10.6 cell engrafted mice, HIV induction was analyzed by comparing GFP expression of J-Lat 10.6 cells in tissues of LRAs treated mice versus the vehicle control group. GFP expression of engrafted J-Lat 10.6 cells was determined by flow cytometry [51].
Histopathological examination
Cell engrafted NSG mice were treated with LRAs. At 24 h after the last dose, mice were sacrificed. Heart, liver, spleen, lung and kidney were collected and fixed in formalin overnight. Tissues were then processed and sectioned for HE staining as described previously [57].
Statistical analysis
All data were analyzed using GraphPad Prism Software (V6) and are presented as means ± the standard error of the mean (SEM). Applied statistical tests and analyses are described in figure legends. P ≤ 0.05 was considered statistically significant. At least three samples were used for each group, the minimum to achieve statistical significance.
Results
EK-16A liposomes reactivate latent HIV in vitro
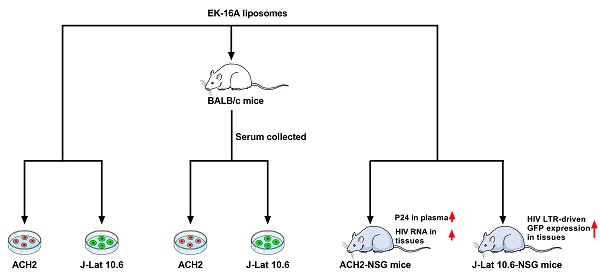
We tested the activity of EK-16A liposomes in vitro. ACH2 cells were treated with EK-16A liposomes for 48 h, the level of P24 antigen in the supernatant was detected by ELISA. Results showed that when the concentration of EK-16A increased from 0 to 4 ng/mL, the level of P24 antigen continued to increase. When the concentration of EK-16A reached 4 ng/ml, the level of P24 antigen reached the plateau, which was 19.74-fold higher than control. EK-16A liposomes induced HIV expression in a dose-dependent manner in the absence of any apparent cellular toxicity in ACH2 cells (Figure 1A and 1B).
The activity of EK-16A liposomes was also verified in J-Lat 10.6 cells. J-Lat 10.6 cells were treated with EK-16A liposomes for 48 h, the proportion of GFP-positive cells was detected by flow cytometry. Results showed that when the concentration of EK-16A liposomes increased from 0 ng/mL to 20 ng/mL, the proportion of GFP-positive cells increased from 5.45% to 63.03%. When the concentration of EK-16A reached 20 ng/ml, the proportion of GFP-positive cells reached the plateau. EK-16A liposomes induced HIV expression in a dose-dependent manner with no effect on cell viability in J-Lat 10.6 cells (Figure 1C and 1D).
Serum from BALB/c mice receiving EK-16A liposomes reactivate latent HIV
In order to explore whether EK-16A can be absorbed into blood and retain activity after oral administration, we performed the serum pharmacological test. EK-16A liposomes were given to mice orally once a day for 3 consecutive days. Blood was collected before treatment and after the last administration. Serum was separated and co-cultured with latently infected cells for 48 h to test whether it can promote the expression of HIV. In the supernatant of ACH2 cells, the level of P24 antigen induced by serum collected from mice receiving 1 mg kg-1 EK-16A treatment at 1 h, 2 h and 3 h after the last administration was respectively 2.92-fold, 4.57-fold and 4.61-fold higher than control. The level of P24 antigen induced by serum collected from mice receiving 0.5 mg/kg EK-16A treatment at 1 h after the last administration was 2.22-fold higher than control (Figure 2A). On J-Lat 10.6 cells, the proportion of GFP-positive cells induced by serum collected from mice receiving 1 mg/kg EK-16A liposomes treatment at 1 h, 2 h and 3 h after the last administration was respectively 20.4%, 32.2% and 33.4%, significantly higher than control, which was 8.77% (Figure 2C). Compared to serum collected before treatment, serum collected after treatment made no apparent cellular toxicity in ACH2 cells (Figure 2B) and J-Lat 10.6 cells (Figure 2D).
Latent HIV reactivation by EK-16A liposomes in vitro. ACH2 or J-Lat 10.6 cells were treated with EK-16A liposomes at the indicated concentrations. (A) HIV expression in ACH2 cells was measured by quantifying the level of p24 in the supernatant and presented as fold induction relative to control. (B) The viability of ACH2 cells was measured using CCK-8 kit. The division of OD450 between treated and control groups indicate the percentage of cell viability. (C) HIV expression in J-Lat 10.6 cells was measured by quantifying the percentage of GFP-positive cells. (D) The viability of J-Lat 10.6 cells was measured as in (C). Data show the means ± SEM. Statistical significance was determined using a student t test, ns p > 0.05, * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001.
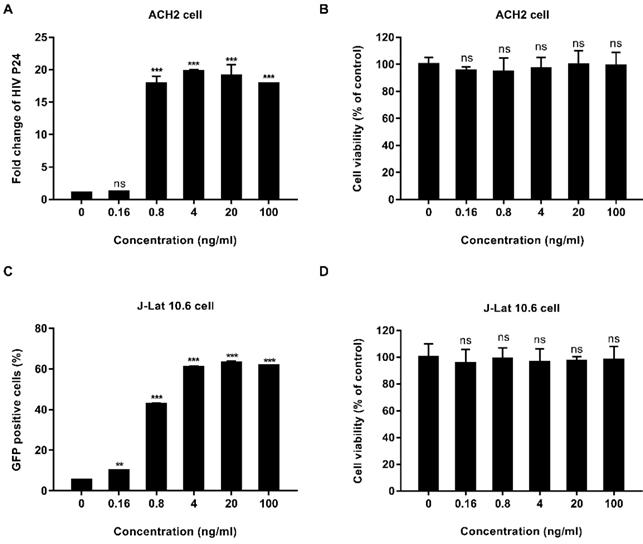
Latent HIV reactivation by serum from BALB/c mice receiving EK-16A liposomes. EK-16A liposomes were administered to BALB/c mice at the dose of 0, 0.5 or 1 mg/kg for 3 days. Before treatment (0 h) and after the last administration for 1 h, 2 h and 3 h, serum was separated and co-cultured with ACH2 or J-Lat 10.6 cells for 48 h. (A and B) In ACH2 cells, the level of p24 antigen in the supernatant (A) and cell viability (B) was analyzed. (C and D) In J-Lat 10.6 cells, the percentage of GFP-positive cells (C) and cell viability (D) was analyzed. N=3, data show the means ± SEM. Statistical significance was determined using a two-way ANOVA test, ns p > 0.05, * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001.
ACH2 and J-Lat 10.6 cell engrafted NSG mouse models are established
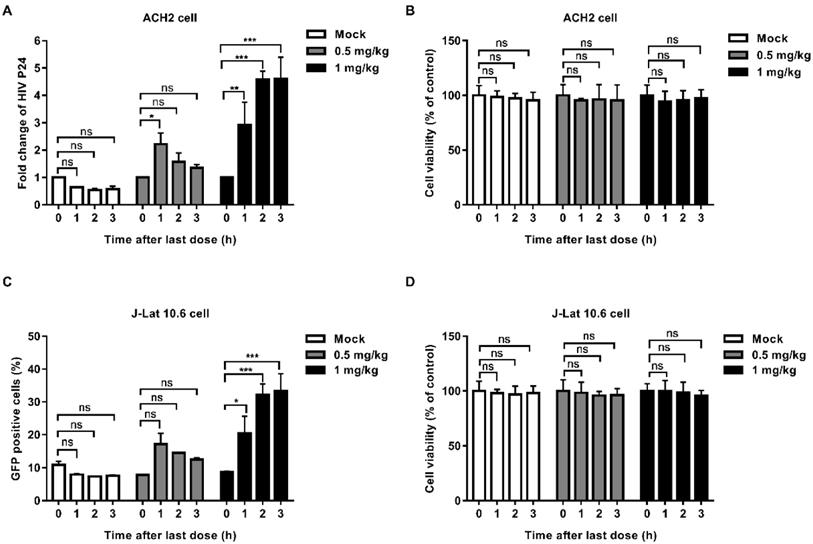
ACH2 cell engrafted NSG mouse model was used for in vivo studies. We first searched for cell surface marker that identified engrafted ACH2 cells in a background of mouse cells. Our candidate panel included six cell surface proteins commonly known to be expressed in human CD4+ T cells: CD147, CD29, HLA-ABC, CD3, CD4 and TCR α β. Three of these cell surface proteins were found to be expressed universally among the ACH2 population: CD147, CD29 and HLA-ABC. The mean fluorescence intensity (MFI) of the proteins, reflecting the relative abundance of each protein on the cell surface, was measured. CD147 exhibited the highest MFI (Figure 3A). We next tested if the CD147 antibodies showed detectable binding to NSG mouse cells obtained from different tissues. While 100% of cultured ACH2 cells expressed (Figure S1A), the frequency of CD147+ cells was negligible or absent across mouse tissues (Figure S1C). Thus, human CD147 could function as a specific marker for the identification of ACH2 cells engrafted in NSG mice.
NSG mice were engrafted intravenously with 1×107 ACH2 cells. The engraftment of ACH2 cells in different tissues was monitored by flow cytometry every week. Results showed that ACH2 cells appeared in the main immune tissues in 3 weeks, and reached the peak in 5 weeks (Figure 3B). The mean frequency of engrafted ACH2 cells varied across tissues. Three weeks post transplantation, it was approximately 43.86% in bone marrow, 0.72% in spleen, 0.40% in liver, 2.17% in lung and 0.22% in peripheral blood. Five weeks post transplantation, it was approximately 79.62% in bone marrow, 29.74% in spleen, 15.03% in liver, 10.04% in lung and 5.51% in peripheral blood (Figure 3C). Notably, starting from the 4th week after ACH2 cell transplantation, weights of mice fluctuated significantly (Figure S2A). Considering both transplantation efficiency and body weight, this model is best used in 3 weeks.
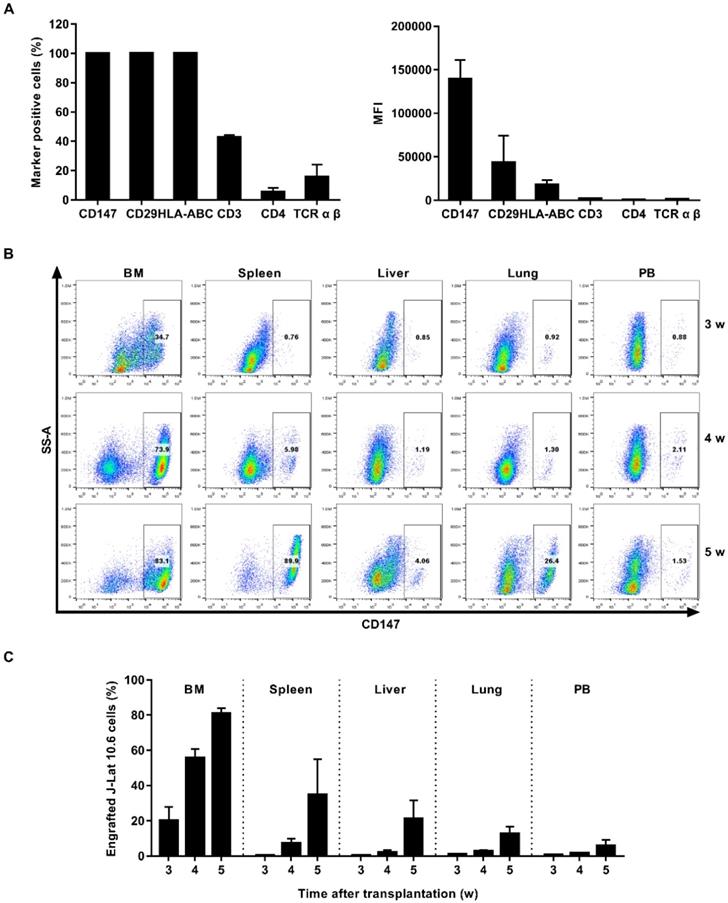
J-Lat 10.6 cell engrafted NSG mouse model was also used. CD147 protein was expressed universally and exhibited the highest MFI among the J-Lat 10.6 population (Figure 4A and Figure S1B). NSG mice were engrafted intravenously with 1×107 J-Lat 10.6 cells. J-Lat 10.6 cells appeared in the main immune tissues in 3 weeks, and reached the peak in 5 weeks (Figure 4B). Three weeks post transplantation, it was approximately 20.32% in bone marrow, 0.55% in spleen, 0.53% in liver, 1.06% in lung and 0.59% in peripheral blood. Five weeks post transplantation, it was approximately 80.86% in bone marrow, 34.84% in spleen, 21.30% in liver, 12.80% in lung and 5.91% in peripheral blood (Figure 4C). Starting from the 4th week after J-Lat 10.6 cell transplantation, weights of mice fluctuated significantly (Figure S2B). Overall, this model is best used in 3 weeks.
Establishment of ACH2 cell engrafted NSG mouse model. (A) ACH2 cells were stained with antibodies targeting select human cell surface, the frequency of marker-positive cells and MFI was measured by flow cytometry. (B) NSG mice were engrafted with ACH2 cells for 3, 4 or 5 weeks, respectively. Representative flow cytometry plots were shown for the engraftment level in tissues. (C) Bar graphs summarized the frequency of engrafted ACH2 cells in respective tissue. N=5, data show the means ± SEM.
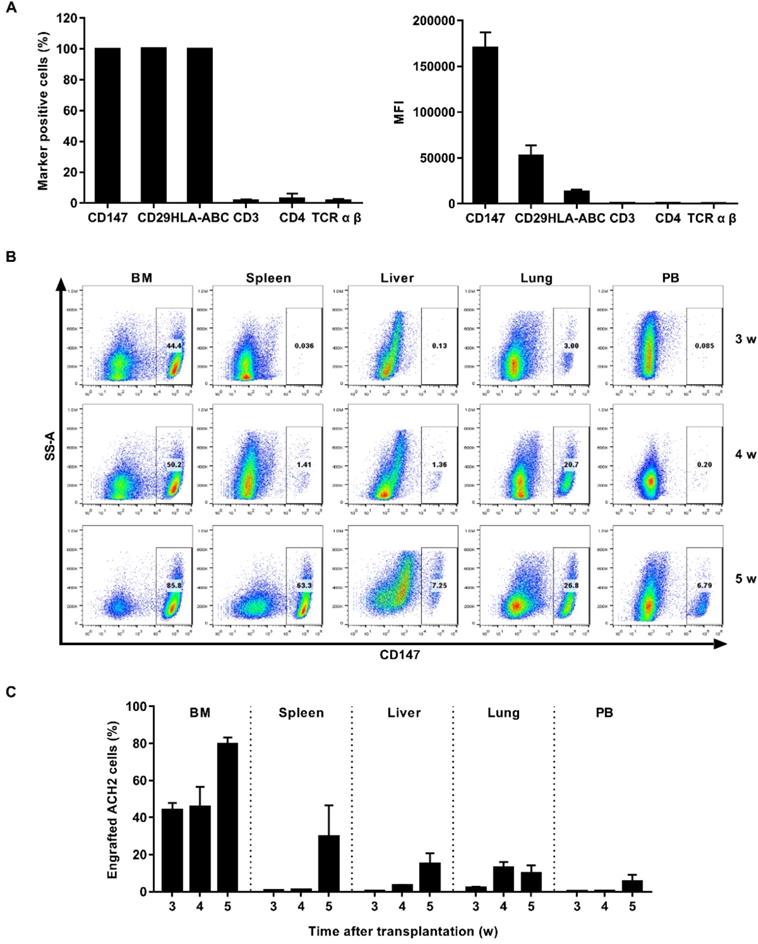
Establishment of J-Lat 10.6 cell engrafted NSG mouse model. (A) J-Lat 10.6 cells were stained with antibodies targeting select human cell surface, the frequency of marker-positive cells and MFI were measured by flow cytometry. (B) NSG mice were engrafted with J-Lat 10.6 cells for 3, 4 or 5 weeks, respectively. Representative flow cytometry plots were shown for the engraftment level in tissues. (C) Bar graphs summarized the frequency of engrafted J-Lat 10.6 cells in respective tissue. N=5, data show the means ± SEM.
EK-16A liposomes enhance HIV replication in cell engrafted NSG mouse models
ACH2 cell engrafted NSG mice were divided into 3 groups and received oral treatment with 1 mg/kg EK-16A liposomes, 60 mg/kg SAHA or vehicle control for 3 days, respectively (Figure 5A). After 3 consecutive EK-16A liposomes treatment, increased P24 antigen secretion was detected in the plasma of mice. However, no change in P24 antigen level was detected in vehicle-control-treated mice nor in SAHA-treated mice (Figure 5B). The hallmark of HIV persistence in humans is the presence of inducible HIV in different tissues. We isolated cells from primary (bone marrow), secondary (spleen), effector (liver and lung) immune tissues and peripheral blood after treatment. The level of HIV RNA from EK-16A-treated mice was significantly higher in bone marrow (2.80-fold), liver (1.46-fold), lung (2.22-fold) and peripheral blood (2.99-fold) compared with vehicle controls, but not in spleen. The level of HIV RNA from SAHA-treated mice was significantly higher in bone marrow (2.06-fold), liver (1.40-fold) and peripheral blood (1.68-fold) compared with vehicle controls, but not in spleen or lung (Figure 5C). Furthermore, no notable difference in histopathological examination was noted among ACH2 cell engrafted NSG mice treated with EK-16A liposomes, SAHA or vehicle control (Figure 7A). Overall, results demonstrate that EK-16A liposomes induce viral reactivation in ACH2 cell engrafted NSG mice.
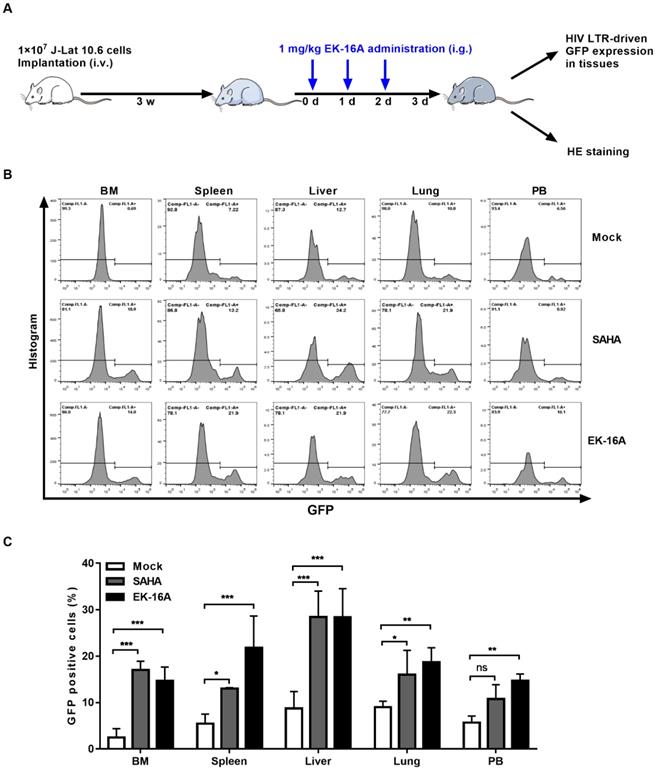
We also evaluated the activity of EK-16A liposomes in NSG mice 3 weeks post J-Lat 10.6 cell transplantation (Figure 6A). Viral reactivation was measured based on the frequency of GFP expressing cells within engrafted J-Lat 10.6 cells from mouse tissues following 3 consecutive treatment. EK-16A liposomes treatment did lead to increase in the frequency of GFP-positive cells, so did SAHA (Figure 6B). Statistics showed that the percentage of GFP-positive cells from EK-16A-treated mice was significantly higher in bone marrow (6.00-fold), spleen (4.00-fold), liver (3.24-fold), lung (2.08-fold) and peripheral blood (2.61-fold) compared with vehicle control. The percentage of GFP-positive cells from SAHA-treated mice was also significantly higher in diverse tissues compared with vehicle control (Figure 6C). There was no significant difference in histopathological examination of mice after treatment (Figure 7B). Taken together, results demonstrate that EK-16A liposomes induce viral reactivation in J-Lat 10.6 cell engrafted NSG mice.
Discussion
Eradication of HIV infection after prolonged viral suppression is the focus of intense research and latency reversal has been a cornerstone of this effort. LRAs have been widely recognized as important tools to induce HIV expression. Future clinical applications of HIV cure strategies must be relevant to the majority of people living with HIV for whom the treatments of the London and Berlin patients [58, 59] pose an unacceptable level of risk. Therefore, LRAs must be identified that are highly effective but have minimal side effects [25].
Enhancing HIV replication by EK-16A liposomes in ACH2 cell engrafted NSG mouse model. (A) Experimental design during EK-16A liposomes treatment phase in ACH2 cell engrafted NSG mice. (B) NSG mouse models were treated with vehicle control, SAHA or EK-16A liposomes for 3 days. The level of P24 in plasma was detected every day and results were presented as fold induction relative to prior treatment for each mouse. (C) After treatment, HIV RNA from cells of respective tissue were analyzed and results were presented as fold induction relative to control. N=5, data show the means ± SEM. Statistical significance was determined using a two-way ANOVA test, ns p > 0.05, * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001.

Enhancing HIV replication by EK-16A liposomes in J-Lat 10.6 cell engrafted NSG mouse model. (A) Experimental design during EK-16A liposomes treatment phase in J-Lat 10.6 cell engrafted NSG mouse model. (B) NSG mouse models were treated with vehicle control, SAHA or EK-16A liposomes for 3 days. Representative flow cytometry histograms were shown for the percentage of GFP positive cells within J-Lat 10.6 cells from respective tissue. (C) Bar graphs summarized the percentage of GFP positive cells within J-Lat 10.6 cells from respective tissue. N=5, data show the means ± SEM. Statistical significance was determined using a two-way ANOVA test, ns p > 0.05, * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001.
EK-16A is an ingenol derivative that extracted from Euphorbia kansui [37]. Our prior studies have suggested that it could reactivate latent HIV by inducing both NF-κB and P-TEFb signaling pathways [38] and meanwhile inhibit HIV infection by down-regulating the expression of cell surface HIV co-receptors CCR5 and CXCR4 [39] in vitro. Here, we further advanced the research of EK-16A. EK-16A is a kind of oily compound with high hydrophobicity and low solubility. It was prepared into liposomes with lecithin and cholesterol to promote oral administration [60, 61]. Two different systems, ACH2 and J-Lat 10.6, were used in the study. The concordance between the results obtained in two systems highlights the reproducible nature of the effect of EK-16A on enhancing HIV replication.
In vitro, EK-16A liposomes promote the secretion of P24 in ACH2 cells and the expression of GFP in J-Lat 10.6 cells, both with a dose-dependent effect. Serum pharmacological test, commonly used in traditional Chinese medicine [46-50], was introduced to prove that EK-16A were still active after being absorbed into blood. When serum was added into cell culture medium, the concentration of EK-16A was diluted 10 times, which resulted in less effective at some low doses.
Histopathological examination of NSG mouse models after EK-16A liposomes treatment. After treated with vehicle control, SAHA or EK-16A liposomes for 3 days, ACH2 cell engrafted NSG mice (A) or J-Lat 10.6 cell engrafted NSG mice (B) were sacrificed. Heart, liver, spleen, lung and kidney were collected and HE staining was performed.

In vivo, by applying widely utilized HIV latently infected cell lines, we achieved the establishment of two mouse models in short time, circumventing the need for donor derived tissues. ACH2 and J-Lat 10.6 cell engrafted NSG mouse models were scalable, accessible, and cost-effective. Although not intended to serve as pathophysiology models, they could be used to evaluate the performance of HIV cure strategies in distinct anatomical niches [51]. SAHA, also known as vorinostat, a histone deacetylase inhibitor, has been widely evaluated as an LRA [28, 29, 31], was served as a control. In ACH2 cell engrafted NSG mice, multiple doses of EK-16A liposomes increased the secretion of P24 antigen in plasma and the expression of cell-associated HIV RNA in diverse tissues. The index of P24 antigen is not as sensitive as that of plasma viral RNA, but it is more convincing [56]. In J-Lat 10.6 cell engrafted NSG mice, multiple doses of EK-16A liposomes increased the expression of GFP in J-Lat 10.6 cells from diverse tissues. Previous attempts to reactivate HIV in preclinical animal models and in clinical trials have measured HIV induction in the peripheral blood with minimal focus on tissue reservoir and have had limited effect [25]. Our results provided in vivo evidence of systemic enhancing HIV replication for EK-16A liposomes. Importantly, there was no obvious histopathological change associated with EK-16A liposomes in the two mouse models. Of course, the safety of EK-16A liposomes needs much more stringent assessment in the future. For example, long-term toxicity experiments should be performed to exclude the possibility of accumulative toxicity in mice due in an extended time duration.
In summary, our results demonstrated the activity and preliminary security of EK-16A liposomes on enhancing HIV replication in human cell engrafted NSG mice, laying the foundation for research in more advanced animal models and clinical trials. The study of EK-16A liposome in vivo indicate that ingenol compound can be possibly tested in a more clinically relevant model of HIV in an appropriate formula. Undeniably, there were still some limitations. The study lacked dedicated “kill” interventions. Combining “shock” interventions with “kill” components is a key next step [62]. This promising compound, in combination with appropriate tools for clearance of HIV infection, may increase opportunities for HIV eradication.
Abbreviations
ART: antiretroviral therapy; BM: bone marrow; ELISA: enzyme linked immunosorbent assay; GFP: green fluorescent protein; HIV: human immunodeficiency virus; LRAs: latency reversal agents; LTR: long terminal repeat; MFI: mean fluorescence intensity; NF-κB: nuclear factor κB; PB: peripheral blood; PBS: phosphate buffer saline; PCR: phosphate buffer saline; PKC: protein kinase C; P-TEFb: positive transcription elongation factor b; SAHA: suberoylanilide hydroxamic acid.
Supplementary Material
Supplementary figures.
Acknowledgements
This work was supported by National Grant Program for Key Infectious Diseases of China (2017ZX10202102002) and National Natural Science Foundation of China (81761128020 and 31771484).
Author Contributions
H.Z. conceived and designed the experiments. P.L. carried out most experiments. J.Y., X.Y., Z.L., J.W., Y.W., L.Z., H.P., X.S. and Y.Z. participated in some of the experiments. H.Z. and H.L. directed and supervised the experiments and interpretation of data. P.L. and H.Z. wrote the main manuscript text. All authors approved the publication.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Gulick RM, Mellors JW, Havlir D, Eron JJ, Gonzalez C, McMahon D. et al. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. The New England journal of medicine. 1997;337:734-9
2. Perelson AS, Essunger P, Cao Y, Vesanen M, Hurley A, Saksela K. et al. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature. 1997;387:188-91
3. Li TS, Tubiana R, Katlama C, Calvez V, Ait Mohand H, Autran B. Long-lasting recovery in CD4 T-cell function and viral-load reduction after highly active antiretroviral therapy in advanced HIV-1 disease. Lancet. 1998;351:1682-6
4. Chun TW, Stuyver L, Mizell SB, Ehler LA, Mican JA, Baseler M. et al. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:13193-7
5. Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, Chaisson RE. et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295-300
6. Wong JK, Hezareh M, Gunthard HF, Havlir DV, Ignacio CC, Spina CA. et al. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1291-5
7. Pitman MC, Lau JSY, McMahon JH, Lewin SR. Barriers and strategies to achieve a cure for HIV. Lancet HIV. 2018;5:e317-e28
8. Zerbato JM, Purves HV, Lewin SR, Rasmussen TA. Between a shock and a hard place: challenges and developments in HIV latency reversal. Curr Opin Virol. 2019;38:1-9
9. Deeks SG. HIV: Shock and kill. Nature. 2012;487:439-40
10. Archin NM, Kirchherr JL, Sung JA, Clutton G, Sholtis K, Xu Y. et al. Interval dosing with the HDAC inhibitor vorinostat effectively reverses HIV latency. J Clin Invest. 2017;127:3126-35
11. Rasmussen TA, Tolstrup M, Brinkmann CR, Olesen R, Erikstrup C, Solomon A. et al. Panobinostat, a histone deacetylase inhibitor, for latent-virus reactivation in HIV-infected patients on suppressive antiretroviral therapy: a phase 1/2, single group, clinical trial. Lancet HIV. 2014;1:e13-21
12. Matalon S, Rasmussen TA, Dinarello CA. Histone deacetylase inhibitors for purging HIV-1 from the latent reservoir. Mol Med. 2011;17:466-72
13. Sogaard OS, Graversen ME, Leth S, Olesen R, Brinkmann CR, Nissen SK. et al. The Depsipeptide Romidepsin Reverses HIV-1 Latency In vivo. PLoS Pathog. 2015;11:e1005142
14. Nguyen K, Das B, Dobrowolski C, Karn J. Multiple Histone Lysine Methyltransferases Are Required for the Establishment and Maintenance of HIV-1 Latency. mBio. 2017 8
15. Li Z, Guo J, Wu Y, Zhou Q. The BET bromodomain inhibitor JQ1 activates HIV latency through antagonizing Brd4 inhibition of Tat-transactivation. Nucleic Acids Res. 2013;41:277-87
16. Lu P, Qu X, Shen Y, Jiang Z, Wang P, Zeng H. et al. The BET inhibitor OTX015 reactivates latent HIV-1 through P-TEFb. Sci Rep. 2016;6:24100
17. Lu P, Shen Y, Yang H, Wang Y, Jiang Z, Yang X. et al. BET inhibitors RVX-208 and PFI-1 reactivate HIV-1 from latency. Sci Rep. 2017;7:16646
18. Jiang G, Dandekar S. Targeting NF-kappaB signaling with protein kinase C agonists as an emerging strategy for combating HIV latency. AIDS Res Hum Retroviruses. 2015;31:4-12
19. Margolis DM, Archin NM, Cohen MS, Eron JJ, Ferrari G, Garcia JV. et al. Curing HIV: Seeking to Target and Clear Persistent Infection. Cell. 2020;181:189-206
20. McBrien JB, Mavigner M, Franchitti L, Smith SA, White E, Tharp GK. et al. Robust and persistent reactivation of SIV and HIV by N-803 and depletion of CD8(+) cells. Nature. 2020;578:154-9
21. Webb GM, Li S, Mwakalundwa G, Folkvord JM, Greene JM, Reed JS. et al. The human IL-15 superagonist ALT-803 directs SIV-specific CD8(+) T cells into B-cell follicles. Blood Adv. 2018;2:76-84
22. Vibholm L, Schleimann MH, Hojen JF, Benfield T, Offersen R, Rasmussen K. et al. Short-Course Toll-Like Receptor 9 Agonist Treatment Impacts Innate Immunity and Plasma Viremia in Individuals With Human Immunodeficiency Virus Infection. Clin Infect Dis. 2017;64:1686-95
23. Macedo AB, Novis CL, Bosque A. Targeting Cellular and Tissue HIV Reservoirs With Toll-Like Receptor Agonists. Front Immunol. 2019;10:2450
24. Vibholm LK, Konrad CV, Schleimann MH, Frattari G, Winckelmann A, Klastrup V. et al. Effects of 24-week Toll-like receptor 9 agonist treatment in HIV type 1+ individuals. AIDS. 2019;33:1315-25
25. Nixon CC, Mavigner M, Sampey GC, Brooks AD, Spagnuolo RA, Irlbeck DM. et al. Systemic HIV and SIV latency reversal via non-canonical NF-kappaB signalling in vivo. Nature. 2020;578:160-5
26. Gay CL, Bosch RJ, Ritz J, Hataye JM, Aga E, Tressler RL. et al. Clinical Trial of the Anti-PD-L1 Antibody BMS-936559 in HIV-1 Infected Participants on Suppressive Antiretroviral Therapy. J Infect Dis. 2017;215:1725-33
27. Wightman F, Solomon A, Kumar SS, Urriola N, Gallagher K, Hiener B. et al. Effect of ipilimumab on the HIV reservoir in an HIV-infected individual with metastatic melanoma. AIDS. 2015;29:504-6
28. Archin NM, Liberty AL, Kashuba AD, Choudhary SK, Kuruc JD, Crooks AM. et al. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature. 2012;487:482-5
29. Archin NM, Bateson R, Tripathy MK, Crooks AM, Yang KH, Dahl NP. et al. HIV-1 expression within resting CD4+ T cells after multiple doses of vorinostat. J Infect Dis. 2014;210:728-35
30. Elliott JH, Wightman F, Solomon A, Ghneim K, Ahlers J, Cameron MJ. et al. Activation of HIV transcription with short-course vorinostat in HIV-infected patients on suppressive antiretroviral therapy. PLoS Pathog. 2014;10:e1004473
31. Fidler S, Stohr W, Pace M, Dorrell L, Lever A, Pett S. et al. Antiretroviral therapy alone versus antiretroviral therapy with a kick and kill approach, on measures of the HIV reservoir in participants with recent HIV infection (the RIVER trial): a phase 2, randomised trial. Lancet. 2020;395:888-98
32. Leth S, Schleimann MH, Nissen SK, Hojen JF, Olesen R, Graversen ME. et al. Combined effect of Vacc-4x, recombinant human granulocyte macrophage colony-stimulating factor vaccination, and romidepsin on the HIV-1 reservoir (REDUC): a single-arm, phase 1B/2A trial. Lancet HIV. 2016;3:e463-72
33. Gutierrez C, Serrano-Villar S, Madrid-Elena N, Perez-Elias MJ, Martin ME, Barbas C. et al. Bryostatin-1 for latent virus reactivation in HIV-infected patients on antiretroviral therapy. AIDS. 2016;30:1385-92
34. Spivak AM, Andrade A, Eisele E, Hoh R, Bacchetti P, Bumpus NN. et al. A pilot study assessing the safety and latency-reversing activity of disulfiram in HIV-1-infected adults on antiretroviral therapy. Clin Infect Dis. 2014;58:883-90
35. Cary DC, Fujinaga K, Peterlin BM. Euphorbia Kansui Reactivates Latent HIV. PLoS One. 2016;11:e0168027
36. Liu Q, Li W, Huang L, Asada Y, Morris-Natschke SL, Chen CH. et al. Identification, structural modification, and dichotomous effects on human immunodeficiency virus type 1 (HIV-1) replication of ingenane esters from Euphorbia kansui. Eur J Med Chem. 2018;156:618-27
37. Hou JJ, Wu WY, Liang J, Yang Z, Long HL, Cai LY. et al. A single, multi-faceted, enhanced strategy to quantify the chromatographically diverse constituents in the roots of Euphorbia kansui. J Pharm Biomed Anal. 2014;88:321-30
38. Wang P, Lu P, Qu X, Shen Y, Zeng H, Zhu X. et al. Reactivation of HIV-1 from Latency by an Ingenol Derivative from Euphorbia Kansui. Sci Rep. 2017;7:9451
39. Yang H, Li X, Yang X, Lu P, Wang Y, Jiang Z. et al. Dual effects of the novel ingenol derivatives on the acute and latent HIV-1 infections. Antiviral Res. 2019;169:104555
40. Cui M, Wu W, Hovgaard L, Lu Y, Chen D, Qi J. Liposomes containing cholesterol analogues of botanical origin as drug delivery systems to enhance the oral absorption of insulin. Int J Pharm. 2015;489:277-84
41. Halper-Stromberg A, Lu CL, Klein F, Horwitz JA, Bournazos S, Nogueira L. et al. Broadly neutralizing antibodies and viral inducers decrease rebound from HIV-1 latent reservoirs in humanized mice. Cell. 2014;158:989-99
42. Clouse KA, Powell D, Washington I, Poli G, Strebel K, Farrar W. et al. Monokine regulation of human immunodeficiency virus-1 expression in a chronically infected human T cell clone. J Immunol. 1989;142:431-8
43. Folks TM, Clouse KA, Justement J, Rabson A, Duh E, Kehrl JH. et al. Tumor necrosis factor alpha induces expression of human immunodeficiency virus in a chronically infected T-cell clone. Proc Natl Acad Sci U S A. 1989;86:2365-8
44. Jordan A, Bisgrove D, Verdin E. HIV reproducibly establishes a latent infection after acute infection of T cells in vitro. EMBO J. 2003;22:1868-77
45. Yang X, Wang Y, Lu P, Shen Y, Zhao X, Zhu Y. et al. PEBP1 suppresses HIV transcription and induces latency by inactivating MAPK/NF-kappaB signaling. EMBO Rep. 2020;21:e49305
46. Umeda M, Amagaya S, Ogihara Y. Effects of certain herbal medicines on the biotransformation of arachidonic acid: a new pharmacological testing method using serum. J Ethnopharmacol. 1988;23:91-8
47. Bochu W, Liancai Z, Qi C. Primary study on the application of Serum Pharmacology in Chinese traditional medicine. Colloids Surf B Biointerfaces. 2005;43:194-7
48. Jiang YR, Miao Y, Yang L, Xue M, Guo CY, Ma XJ. et al. Effect of chinese herbal drug-containing serum for activating-blood and dispelling-toxin on ox-LDL-induced inflammatory factors' expression in endothelial cells. Chin J Integr Med. 2012;18:30-3
49. Lu J, Zhang X, Shen T, Ma C, Wu J, Kong H. et al. Epigenetic Profiling of H3K4Me3 Reveals Herbal Medicine Jinfukang-Induced Epigenetic Alteration Is Involved in Anti-Lung Cancer Activity. Evid Based Complement Alternat Med. 2016;2016:7276161
50. Iwama H, Amagaya S, Ogihara Y. Effect of shosaikoto, a Japanese and Chinese traditional herbal medicinal mixture, on the mitogenic activity of lipopolysaccharide: a new pharmacological testing method. J Ethnopharmacol. 1987;21:45-53
51. Sperber HS, Togarrati PP, Raymond KA, Bouzidi MS, Gilfanova R, Gutierrez AG. et al. mu-Lat: A mouse model to evaluate human immunodeficiency virus eradication strategies. FASEB J. 2020;34:14615-30
52. Kuai Q, Wang Y, Gao F, Qi Y, Wang R, Wang Y. et al. Peptide Self-Assembly Nanoparticles Loaded with Panobinostat to Activate Latent Human Immunodeficiency Virus. J Biomed Nanotechnol. 2019;15:979-92
53. Wang Y, Wang J, Yang X, Yang J, Lu P, Zhao L. et al. Chemokine Receptor CCR2b Enhanced Anti-tumor Function of Chimeric Antigen Receptor T Cells Targeting Mesothelin in a Non-small-cell Lung Carcinoma Model. Front Immunol. 2021;12:628906
54. Zhang H, Li F, Cao J, Wang X, Cheng H, Qi K. et al. A chimeric antigen receptor with antigen-independent OX40 signaling mediates potent antitumor activity. Sci Transl Med. 2021 13
55. Beyer AI, Muench MO. Comparison of Human Hematopoietic Reconstitution in Different Strains of Immunodeficient Mice. Stem Cells Dev. 2017;26:102-12
56. Bullen CK, Laird GM, Durand CM, Siliciano JD, Siliciano RF. New ex vivo approaches distinguish effective and ineffective single agents for reversing HIV-1 latency in vivo. Nat Med. 2014;20:425-9
57. Cao S, Slack SD, Levy CN, Hughes SM, Jiang Y, Yogodzinski C. et al. Hybrid nanocarriers incorporating mechanistically distinct drugs for lymphatic CD4(+) T cell activation and HIV-1 latency reversal. Sci Adv. 2019;5:eaav6322
58. Gupta RK, Abdul-Jawad S, McCoy LE, Mok HP, Peppa D, Salgado M. et al. HIV-1 remission following CCR5Delta32/Delta32 haematopoietic stem-cell transplantation. Nature. 2019;568:244-8
59. Hutter G, Nowak D, Mossner M, Ganepola S, Mussig A, Allers K. et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med. 2009;360:692-8
60. Nguyen TX, Huang L, Gauthier M, Yang G, Wang Q. Recent advances in liposome surface modification for oral drug delivery. Nanomedicine (Lond). 2016;11:1169-85
61. Daeihamed M, Dadashzadeh S, Haeri A, Akhlaghi MF. Potential of Liposomes for Enhancement of Oral Drug Absorption. Curr Drug Deliv. 2017;14:289-303
62. Lichterfeld M. Reactivation of latent HIV moves shock-and-kill treatments forward. Nature. 2020;578:42-3
Author contact
![]() Corresponding author: Huanzhang Zhu. E-mail: hzzhuedu.cn
Corresponding author: Huanzhang Zhu. E-mail: hzzhuedu.cn

 Global reach, higher impact
Global reach, higher impact