ISSN: 2206-7418
Nanotheranostics 2022; 6(4):424-435. doi:10.7150/ntno.76370 This issue Cite
Research Paper
Stealth Liposomal Chemotherapeutic Agent for Triple Negative Breast Cancer with Improved Pharmacokinetics
1. Nanomedicine and Advanced Drug Delivery Lab, Department of Pharmaceutics, National Institute of Pharmaceutical Education and Research (NIPER), Hyderabad, Telangana, India - 500037.
2. Drug Metabolism and Interactions Research Lab, Department of Pharmaceutical Analysis, National Institute of Pharmaceutical Education and Research (NIPER), Hyderabad, Telangana, India - 500037.
3. Department of Pharmaceutical Analysis, St. James College of Pharmaceutical Sciences (SJCOPS), Chalakudy, Kerala, India - 680307.
4. Department of Natural Products, National Institute of Pharmaceutical Education & Research (NIPER) Kolkata, Chunilal Bhawan, Maniktala, Kolkata, West Bengal, India - 700054.
Abstract
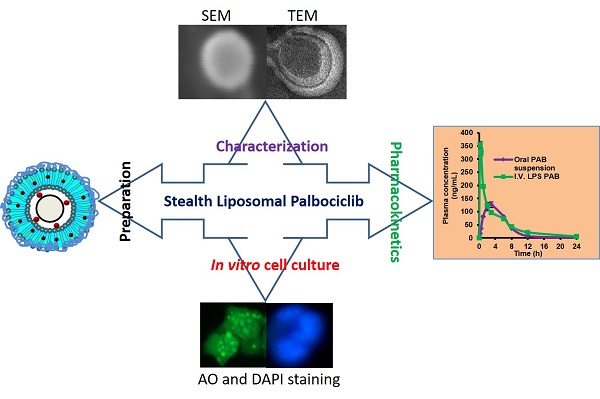
Triple-negative breast cancer is one of the most lethal cancers. Chemotherapeutics for targeting CDK4 and CDK6 like Palbociclib (PAB) in triple-negative breast cancer was widely explored. However, poor bioavailability and severe side effects profile limiting its clinical usage in the field of cancer chemotherapy. Herein, we set out to develop the stealth liposomes (LPS) of PAB by rotary thin film evaporation with a vesicle size of less than 100 nm. In vitro, drug release studies were performed and fitted into different release kinetic models. LPS were characterized by electron microscopic techniques for morphology. The engineered nanotherapeutics agents were further evaluated in 4T1 triple-negative breast cancer cell lines for its anti-cancer potential and cellular uptake. The hemolytic potential and pharmacokinetic (PK) behavior of developed LPS-PAB and PAB were analyzed by using robust UHPLC-QTOF-MS method. LPS-PAB demonstrates biphasic release profile with first-order release kinetics. Further, LPS-PAB has shown less IC50 value (1.99 µM) compared to PAB alone (3.24 µM). The designed nanoliposomes were tagged with fluorescent FITC dye to check rapid cellular uptake. Importantly, stealth LPS-PAB has shown a 1.75-fold reduction in hemolytic potential as compared to PAB plain drug at 100 µg/mL concentration. The PK results obtained was displayed 2.5-fold increase in Cmax, 1.45-fold increase in AUCtot, 1.8-fold increase in half-life and 1.3-fold increase in MRT with LPS-PAB when compared to orally administered PAB suspension. These findings suggest that novel LPS-PAB can be employed as an alternate therapeutic strategy to eradicate triple-negative breast cancer.
Keywords: Liposomes, Palbociclib, Pharmacokinetics, PEGylation, Triple Negative Breast Cancer

 Global reach, higher impact
Global reach, higher impact