ISSN: 2206-7418
Nanotheranostics 2022; 6(4):436-450. doi:10.7150/ntno.73918 This issue Cite
Review
Progress on Applying Carbon Dots for Inhibition of RNA Virus Infection
1. Department of Chemistry, Pakokku University, Myaing Road, Pakokku 90401, Myanmar.
2. Department of Chemistry, University of Miami, Coral Gables, FL 33146, USA.
3. Department of Chemistry, Universitas Airlangga, Surabaya 60115, Indonesia.
4. Supramodification Nano-micro Engineering Research Group, Universitas Airlangga, Surabaya 60115, Indonesia.
5. Research Director at Walchand Center for Research in Nanotechnology and Bionanotechnology, Walchand College of Arts and Science, W. H. Road, Ashok Chowk, Solapur 413006, India.
Received 2022-4-12; Accepted 2022-8-11; Published 2022-8-21
Abstract
Viral infection is a globally leading health issue. Annually, new lethal RNA viruses unexpectedly emerged and mutated threatening health and safety. Meanwhile, it is urgent to explore novel antiviral agents, which, however, takes years to be clinically available. Nonetheless, the development of carbon dots (CDs) in the past 20 years has exhibited their vast application potentials and revealed their promising capacity as future antiviral agents considering their versatile properties and significant antiviral responses. Thus, CDs have been widely investigated as an alternative of traditional chemotherapy for inhibiting viral infection and replication in vitro. Meanwhile, attempts to apply CDs to in vivo systems are in high demand. In this review, recent developments of CDs-based antiviral therapies are systematically summarized. Furthermore, the role of CDs in photodynamic inactivation to kill viruses or bacteria is briefly discussed.
Keywords: RNA virus, virus infection treatment, carbon dots, nanomedicine, antiviral therapy
Introduction
An RNA virus indicates a virus that has RNA as its genetic material. Notable human diseases that result from RNA virus infection include the common cold, influenza, SARS, COVID-19, MERS, hepatitis C, E, West Nile fever, Ebola virus disease, rabies, mumps and acquired immune deficiency syndrome (AIDS) [1]. Although the genetic material of a virus can be either DNA or RNA, RNA viruses generally have higher mutation rates since viral RNA polymerases lack the proofreading ability as DNA polymerases [2], which explains why it is difficult to create effective vaccines against RNA viruses [3]. As a typical RNA virus model, since the 1980s, the human immunodeficiency virus (HIV) has infected 33 million people all over the world. Meanwhile, there have been more than 3 million new cases and 2 million deaths each year ever since [4]. And the COVID-19 since its break out in 2019, globally, as of 6:34 pm CEST, 28 July 2022, there have been 571,198,904 confirmed cases of COVID-19, including 6,387,863 deaths, reported to WHO. Thus, it is very urgent and significant to solve these health problems induced by various RNA virus infections.
In terms of antiviral therapy, currently, drug delivery systems (DDS) combined with highly active antiretroviral therapy (HAART) is a common practice. It increases the longevity of HIV-infected patients. However, some challenges still remain [5].
Despite the great advances in technology for virus management, viruses cannot be eradicated by current treatment of HAART due to considerable restrictions and the main challenge during the course of treatment is poor patient compliance in the long run, which usually leads to a poor therapeutic efficacy and requires a longer treatment period [6]. Hence, an alternative nanotechnological approach has timely emerged as a scientifically potential solution in a short-term pursuit of an effective therapeutic strategy against HIV and other RNA viruses, such as dengue virus (DENV), Ebola virus (EBOV), and pulmonary syndrome corona [7, 8].
Nanotechnology has tremendous potential to fight against viruses with nanoparticles (NPs) [9]. Those nanoscale particles are able to penetrate small tissue systems and play as prominent nanomedicine or drug nanocarriers for the treatment of various viral diseases and cancers [10]. The antiviral activity of NPs has been standardized through various mechanisms to provide their antiviral mode of action: small particle size, high surface areas to volume ratios, tuneable surface electric charge, which will benefit their drug delivery, drug loading, and cell penetration, respectively [11-13].
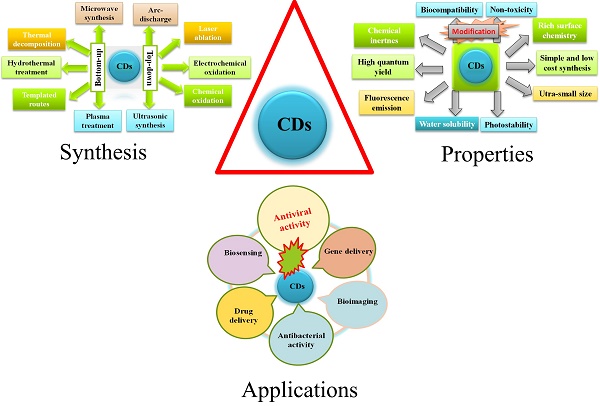
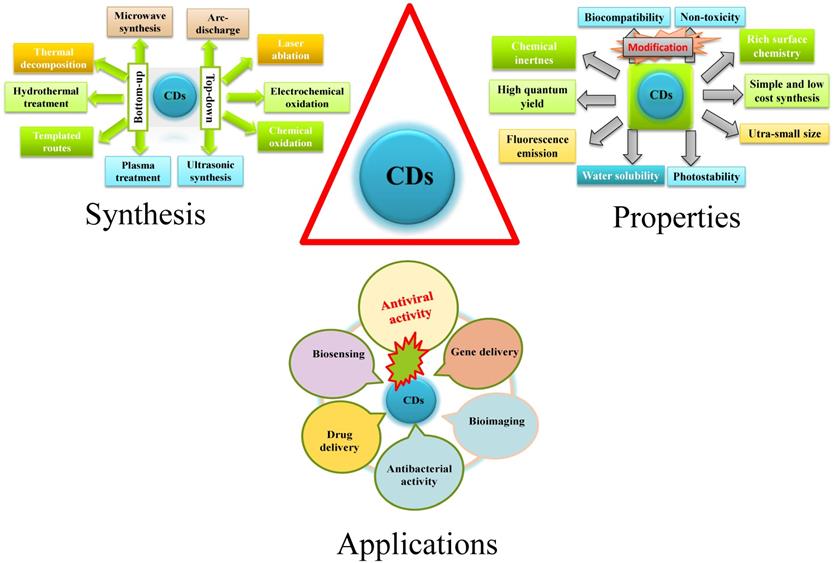
A general schematic diagram of synthesis, properties, and applications of CDs.
Additionally, encapsulation, surface functionalization, and structural modifications can optimize drug dosing, which enhances delivery towards anatomically privileged cellular sites, specific targeted tissues, or subcellular compartments [14]. Moreover, nanotechnology platforms for diagnosis of RNA viruses consist of polymer NPs [15], liposomes [16], dendrimers [17], nano-emulsions [18], and nanosuspensions [19]. These nanomaterials have been applied to make considerable efforts to fight viral threats to some limited level but their water solubility, patient adherence, biostability, and bioavailability need to be further improved [20].
Furthermore, NPs have been synthesized for HIV and other RNA viruses' inhibition such as noble metal-attributed drug-delivery agents [21], polymers [22], composite materials [23], and lipids [24]. However, uses of these NPs are limited by their toxicity, inert properties of starting material, colloidal instability, and complicated synthesis procedures [25]. Therefore, many nanomaterials have been developed, such as carbon dots (CDs), to address all the aforementioned issues while diminishing these health problems around the world.
CDs have exhibited excellent antiviral activities due to some aforementioned properties and high affinity of multifunctional groups to various specific cellular sites [26]. For instance, the outer shell of HIV virus is composed of two layers of lipids with multiple proteins spikes including gp120 and gp41 glycoproteins [27]. The binding between HIV and T-cells is due to the interaction between gp120 and CD4 receptors on T cells. CDs with certain surface functional moieties attract those spike proteins of gp120 receptors, and thus CDs can be bound to HIV. Consequently, the use of CDs will inhibit viral entry into host cells. There are some literature review articles in terms of nanotechnology-approach-derived nanomedicine for RNA viruses' theranostics [28-33]. Apart from these strategies, the current review focuses on the nanotechnology where CDs were shown as powerful antiviral agents against RNA viruses. Eventually, the current review aims to largely outline the potential of CDs in enhanced virus prevention and treatment.
Syntheses of Carbon Dots
CDs, a scientifically interesting class of carbon-based nanomaterial with a size below 10 nm, were unexpectedly discovered when single-walled carbon nanotubes (SWCNTs) were purified through electrophoresis method in 2004 [34, 35]. In 2006, Sun et al. prepared CDs from finely ground graphite and cement dust particles through laser ablation [36]. The synthetic approaches of CDs generally include top-down and bottom-up approaches. The top-down approach covers acid dehydration, laser ablation of graphite, laser passivation, plasma treatment, electrochemical or chemical oxidation, and arc discharge on diverse carbon-based starting materials. On the contrary, the bottom-up approach includes pyrolysis, thermal carbonization, microwave or ultrasonic treatments, and hydrothermal/solvothermal treatments on various small organic molecules or compounds [37, 38]. Biogenic synthesis of CD using of biological entities as precursors such as microbes, fungi, plant extracts, plant metabolites and secretes, and also metabolites of the animal; have become more prevalent for its use in biosystem.
Properties of Carbon Dots
CDs exhibit many outstanding merits including excellent water solubility/dispersity, chemical inertness, good biocompatibility, low toxicity, cost‐effective synthesis, good elasticity for surface modifications, photoluminescence (PL) and good photostability against photo-bleaching [39]. They are extensively applied in the fields of bio‐imaging enhancement [40], bio‐sensing [41], photodynamic therapy [42] photocatalysis [43], thermoelectricity [44], hybrid rocket fuel [45], drug-delivery [46], antimicrobial [47, 48], and virus inhibition [49]. The PL mainstream mechanism of CDs is ascribed to quantum confinement effect, surface state and molecular state. The fluorescence intensity could be varied with respect to the emission turned from ultraviolet to near infrared (NIR). The fluorescence emission of CDs in the NIR wavelength range is effective for deep tissue penetration [50]. In addition, CDs' surfaces are functionalized by miniature organic compounds, metals, and polymers so that their water dispersibility and nano fluorescence were enhanced [51]. Furthermore, currently, nanoscale functional materials have emerged as novel antiviral agents because of their unique chemical and physical properties. In a nutshell novel and unique properties of CD that have given impetus to its use in theranostics are its's crystallinity, emission property, and electrical conductivity. However, little information is available about the potential effects of CDs on viruses.
Mechanisms of Antiviral Action of Carbon Dots
Theoretically speaking, the spike proteins on viruses interact with cell membrane receptor proteins to enter host cells via endocytosis and rapidly replicate inside host cells. Therefore, the receptor proteins on host cells and the viruses' spike proteins are the main objects to target in the regulation of viral entry. Meanwhile, based on literature records, CDs play key roles in combating viral entry and replication with different mechanisms at different steps of viral infection that include attachment, penetration, replication, and budding. Recent efforts have shown that CD derived from biogenic sources like curcumin, glycyrrhizin, etc. are more promising because of their biocompatibility. Moreover, surface passivation/functionalization of CDs with positive charge and more hydrophilic groups on surface will not only help in understanding the mechanism of antiviral activity of CD, it will also enhance the anti-viral action, reduce the toxicity, so as to go ahead with in vitro as well as in vivo antiviral activity.
Viral Inhibition at Attachment and Penetration Stages
The primary step of virus infection is viral attachment to the host cell, thus hindrance to this step will inactivate the virus. The mechanism to inhibit viral attachment involves suppression of the binding of gp120 on HIV viruses to CD4, a receptor protein, and co-receptors (CCR5 or CXCR4) on T cells, thereby blocking the viral entry process. However, suppressing the binding between viruses and host cells has a weakness that might lead to failure of disinfection when NPs disengage from the surfaces of either viruses or host cells [52].
Fahmi and co-workers applied CDs as a viral entry inhibitor to prevent attachment of gp 120 of HIV viruses to CD4 or co-receptor CCR5 on host cells (Figure 2). [26] The CDs were synthesized with citric acid (CA) as a sole precursor via pyrolysis. A graphene-like structure was observed on the CDs with abundant functional groups such as -OH, -COOH on the edge. To enhance virus inhibition, the prepared CDs were conjugated with carboxyl phenylboronic acid (CBBA). In this case, the boronic acid-modified CDs could bind to viral spikes and thus improve blockage of viral entry. In comparison, the antiviral activity of CBBA-modified CDs displayed an enhancement in inhibiting HIV infection than unmodified CDs. As to their cytotoxicity assessments, the average percentage of cell viabilities at different concentrations of CDs and CBBA-CDs were higher than 80%, revealing both are non-toxic to human cells. Their findings highlighted that the intrinsically antiviral property of boronic acid-modified CDs could be used in HIV prevention and treatment [26].
A graphitic illustration of conjugating CBBA to CDs and mechanism of viral entry inhibition. Reprinted with the permission of the reference [26].

Mechanism of inhibition of viral entry by 4-aminophenylboronic acid hydrochloride derived CDs. Reprinted with permission from the reference [53].
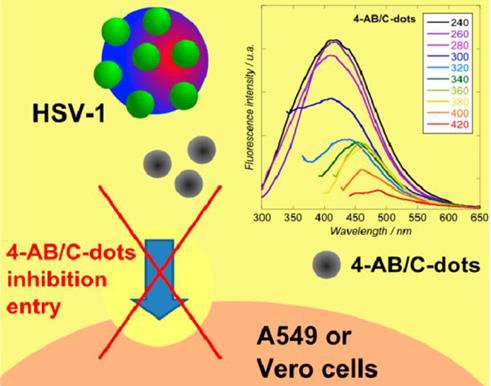
Moreover, Barras et al. [53] investigated the potential of surface-functionalized CDs with boronic acid or amine to prohibit the viral entry of herpes simplex virus type 1 (HSV-1) as depicted in Figure 3. They implied that abundant ligands on the edge of nanostructure of CDs enhanced affinities toward targeted receptors. It was found that the prepared CDs inhibited HSV-1 infection in the concentration range of nanograms per millilitre (EC50 = 80 and 145 ng mL-1 on Vero and A549 cells, respectively) acting on the early stage of viral entry process through an interaction between CDs and the viruses and probably the cells at the same time [53].
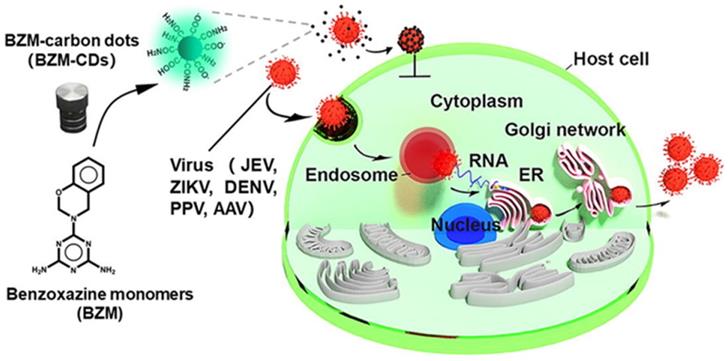
Another study reported by Huang et al. in 2019 was related to the CDs (BZM-CDs) synthesized from a series of benzoxazine monomer. BZM-CDs showed their virus-blocking activity in case of death-dealing with flaviviruses (Japanese encephalitis, dengue viruses, and Zika) and non-enveloped viruses (porcine parvovirus and adenovirus-correlated virus) in vitro as described in Figure 4. It was observed that BZM-CDs immediately adhered to the virion surface, and the initiative of virus-cell interconnection was destroyed by those CDs. It was confirmed that the BZM-CDs could contribute to a fascinating broad-spectrum technique to suppress viral infections [54].
Moreover, based on the aforementioned study, Aung et al. in 2020 fabricated amino phenylboronic acid-modified CDs (APBA-CDs) and employed the newly modified CDs as a novel antiviral agent targeting gp 120 and inhibiting HIV-1 viral entry process (Figure 5) [55]. Their finding demonstrated that in terms of the mechanism of antiviral action, boronic acid-modified APBA-CDs more likely interacted with HIV-1 instead of host cells. It was presumed that the interaction between gp 120 and boronic acid strengthened the attachment of HIV-1 to CDs. In this regard, the important role of boronic acid was highlighted for blocking the viral entry of HIV-1. In addition, the boronic acid on APBA-CDs possibly formed chemical bonding with gp120, which could further disrupt viral contagion. Those boronic acid-modified CDs attracted amino moieties of reverse transcriptase containing HIV and prevented the reverse transcriptase from taking effect in the viral life cycle. Those APBA-CDs showed remarkable biocompatibility, low cytotoxicity (the CC50 reached up to 11.2 mg mL-1) and an excellent potential in inhibiting HIV-1 entry into specifically targeted cells [55].
Virus Inhibition by Ceasing Replication Stage
Once the virus enters the host cell, strategies for interference include prevention of viral replication or budding. Inhibition of viral replication can be achieved by changing enzymes which are required for viral genome replication.
The role of triazole functionalized heteroatom co-doped carbon dots against human coronaviruses i.e. SARS-CoV-2 could be explored. Functionalization of nanomaterials is an important principle for highly anticipated outcomes to producing antiviral agents through both non-covalent bonding (hydrophobic interactions, electrostatic interactions, and π-π stacking) and covalent functionalization strategies. For example, the in-progress potential of triazole-based CDs template was proposed using a series of bioisosteres as antiviral agents to treat human coronavirus infection. CDs have a lot of hydrophilic functional groups on the edges that make them suitable for diverse biomedical applications. The surface functionality of CDs is vital to tune the level of interactions with the viruses [56].
The graphical abstract framework of the prepared BZM-CDs to block viral infectivity. Reproduced with the permission from the reference [54].
The schematic representation of the synthetic process of APBA-CDs via carbonization and function of APBA-CDs in blocking HIV-1 infection to MOLT-4 cells. Reprinted with permission from the reference [55].

Mechanism of viral inhibition of cationic curcumin-derived CDs. Reprinted with permission from the reference [57].
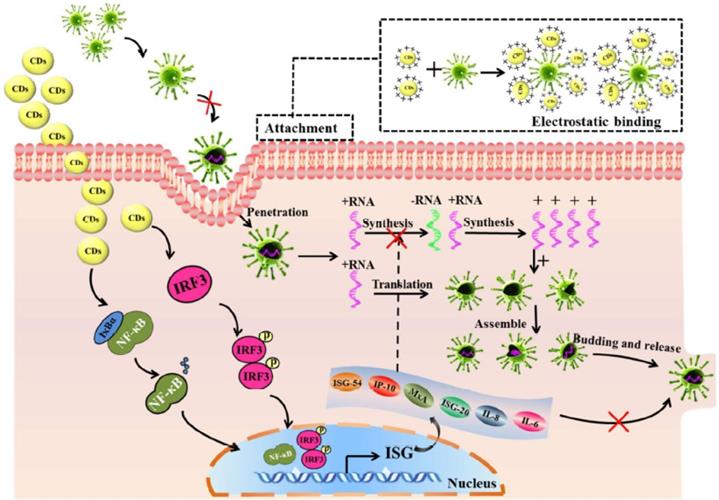
Furthermore, Ting et al. in 2018 found curcumin-derived CDs could markedly knock down the production of negative RNA strand in porcine epidemic diarrhea virus (PEDV), which was proved by the decrease of negative-strand RNA in curcumin-derived CD-treated Cells in contrast with untreated control at various time intervals after viral infection (Figure 6) [57]. The replication of PEDV in Vero cells showed decreasing plaque numbers and reduced virus titers in the CD-treated group compared to the control group [57].
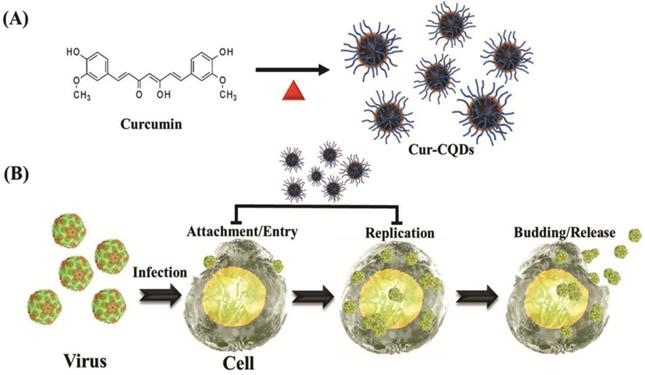
(A) The schematic diagram of the one-step synthesis of Cur-CDs. (B) The general abstract framework of combating viruses with Cur-CDs. Reprinted with permission from the reference [59].
Łoczechin et al. in 2019 prepared functional CDs using ethylenediamine/citric acid as precursors and postmodified the CDs with boronic acid to prevent viral replication of Human Coronavirus [58]. The mechanism of action of those CDs was found to be prevention of HCoV-229E entry which was likely due to interaction between surface functional groups of the CDs and HCoV-229E entry receptors; intriguingly, a comparably high inhibition activity of the CDs was evaluated at the viral replication step [58].
Lin et al. in 2019 also reported antiviral curcumin based-CDs (Cur-CDs) prepared using a one-step dry-heating handling of curcumin inhibited enterovirus 71 (EV71) as shown in Figure 7. The high biocompatibility of Cur-CDs was demonstrated via in vitro cytotoxicity and hemolysis assays. Regulations of synthesis temperature could highly enhance the chemical structural properties of Cur-CDs and their antiviral potency. Their investigation showed that Cur-CDs inhibited EV71 mainly by binding to the virus to prevent viral attachment to the host cell. Furthermore, Cur-CDs could inhibit the viral replication process and prevent the EV71-induced termination of host translation, suggesting that the highly biocompatible Cur-CDs would be a potential agent for therapeutic use against EV71 infection [59].
Viral Inactivation for Preventing Budding and Detachment Stages
Once the virus replicates, the offspring will bud-off from the host cell as new viruses. The plan of actions which can inhibit the budding and excision of more hazardous viruses can also control the viral infection. Viral contagions can be cured with reactive oxygen species (ROS) that are able to damage DNA via apoptotic controlling signals [57].
CDs-based antiviral agents for preventing virus's budding and detachment were found to be of little information; a few other CDs for anti-viral strategies are only explained to provide more information. Three or four compounds combined to formulating CDs have been observed for a highly antiviral activity against herpes simplex virus (HVS-1). According to the viral protein confirmation, the prepared nanocomposites significantly suppressed the infection of HVS-1 under satisfactorily non cytotoxic concentration. And the mechanism for nanostructures to treat HVS-1 was that they prevented the synthesis of viral negative-strand RNA and viral budding [60].
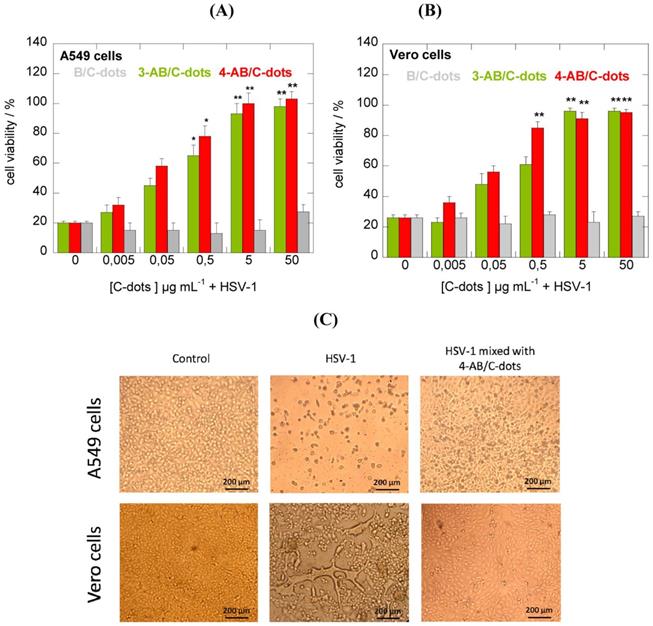
Recent studies have shown the encouraging results to inhibit virus's budding and detachment from the work of nanocomposites modified with boronic acid [61]. For example, CDs have been experimented towards Herpes simplex Virus Type 1 (HSV-1) with potential outcomes in vitro studies on monkey kidney cancer cells (Vero) and human lung cancer cells (A549). According to cytotoxicity tests and antiviral assays of formulated CDs from phenylboronic acid (PBA), 3-aminophenylboronic acid (3-APBA), the formation of multiple functional groups of CDs guarantees the effectiveness against virus infection. Interestingly, the role of CDs can be revealed by a morphological study of the cells. Only boronic acid could not inhibit the HSV-1 in comparison to some nanostructures functionalized with boronic acid. Regarding the cytotoxicity study, it was found that prepared CDs were non-cytotoxic towards A549 cells at concentrations of up to 300 μg mL-1. As shown in Figure 8, it didn't show no infection, showing 100% cell viability at higher dose than 5 μg mL-1 of two prepared 3-APBA/CDs and 4-APBA/CDs [53].
(A) CDs inhibition activities of HSV-1 on A549. (B) Vero cells at varied concentrations. (C) Morphological characteristics of CD effects. Reproduced with permission from the reference [53].
Although three main antiviral mechanisms have been widely found in the use of CDs to treat various viruses, their antiviral activities have also been shown in absence of any presented specific mode of action. Further research is required for investigation of all the plausible modes of strong action of CDs in deactivating, suppressing and killing various RNA viruses.
Antiviral Activity of Carbon Dots against HIV and other RNA Viruses
As precursors of CDs, some natural products including curcumin, flavonoids and polyphenol compounds showed their intrinsic antiviral, antioxidant, anticancer, anti-inflammatory, and antibacterial functions [59]. Ali et al. claimed that curcumin prevents HIV-1 contagion by degrading Tat protein and decreasing the Tat-interposed transcription of the toll-like receptor (TLR) promoter [62]. However, they have poor water-solubility in physiological media and low bioavailability in vivo systems. So, the use of pure natural products to direct cell lines is limited. In this regard, sustainable, safe, and effective alternatives of these compounds transformation into CDs are urgently required to enhance water-solubility, bioavailability, and antiviral ability due to their unique biological properties, morphological characteristics (e.g. controllable size, structure), and physicochemical characteristics which distinctively differs from those of conventional small-molecule dyes or drugs. Therefore, nontoxic CD-base antiviral agents (non-metallic NPs or sustainable nanomaterials) have highly being recommended owing to their water solubility, biocompatibility or safety, high fluorescence, bioavailability, and synergistic antiviral effect [63, 64]. Furthermore, surface modification of CDs has been recently recognized as a cost-effective therapeutic strategy to improve their inhibition towards viral attachment and entry. Ligands present on the surface of CDs could noticeably enhance affinities to multiple cell receptors [53, 58].
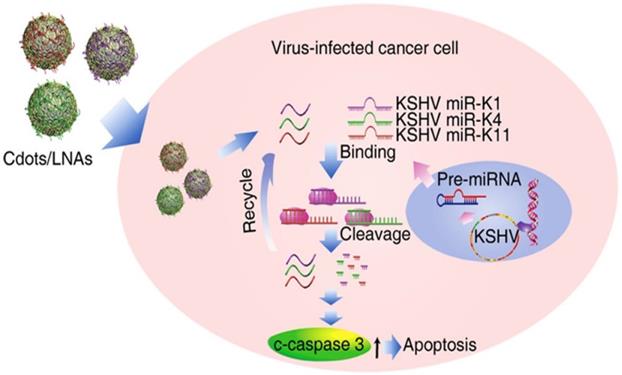
Graphical illustration of viral microRNAs inhibition by CD-interposed drug-loading of LNA for viral therapy. Reproduced with permission from the reference [66].
Dong et al. in 2017 applied antiviral CDs prepared from different surface passivation molecules, namely 2,2′-(ethylenedioxy)bis(ethylamine) and 3-ethoxypropylamine, to inhibit noroviruses [65]. The result showed both CDs significantly inhibited both strains of VLP (virus-like-particles) by binding to histo-blood group antigen (HBGA) receptors on human cells, implying effective inhibition of VLP's binding to HBGA receptors [65].
Ju et al. in 2020 prepared CDs using poly (ethylenimine) and citric acid with microwave-assisted pyrolysis, [66] and the as-prepared CDs were conjugated with locked nucleic acid (LNA) as shown in Figure 9. It was found CD-mediated delivery of locked nucleic acid (CDs-LNA) inhibited the propagation of Kaposi's sarcoma-associated herpesvirus (KSHV) toward initial effusion lymphoma (PEL) cells, inducing apoptosis. The result showed that CDs-LNAs were feasible to deliver model virus suppressors for targeting virus cancers [66].
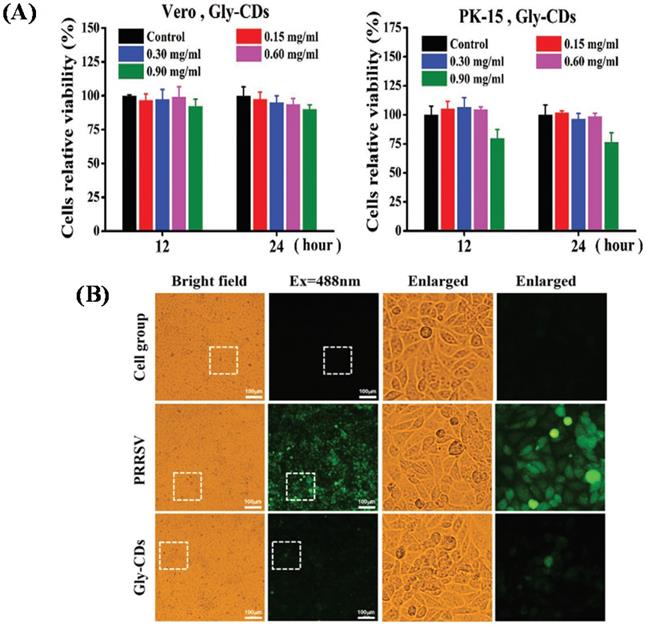
Tong et al. in 2020 prepared glycyrrhizin acid-based CDs (Gly-CDs) from Chinese herbal medicine via hydrothermal treatment. The result indicated that Gly‐CDs could inhibit the porcine propagation and respiratory syndrome virus (PPRSV) invasion and reproduction by stimulating antiviral innate immune systems. The viabilities of both Vero and PK cells make it possible to apply them in biomedical fields considering that the mean percentage of their proliferation rate is above 80% as depicted in Figure 10A. Gly-CDs also inhibited the aggregation of intracellular ROS affected by PRRSV infection as indicated in Figure 10B so they were considered as a potential therapeutic agent for alternative theranostics of PRRSV contagion [67].
Moreover, the use of graphene quantum dots (GQDs) has to be mentioned as an antiviral strategy to harmonize CDs positioning on virus inhibition since GQDs are one of family members of CDs and both of them share multiple superior virtues. CDs stand for an extensive group of carbon-based nanoparticles, their structures are still far from being fully understood. Typically, carbon-dominated nanomaterials are CDs, amorphous carbon nanoparticles, partially graphitized core-shell carbon nanoparticles, amorphous fluorescent polymeric nanoparticles and graphene quantum dots (GQDs). All of them belong to carbon-based fluorescent nanomaterials but their sizes are different [68]. Many graphene lattices which are found in the inner part of GQDs are generated from graphene-based precursors or the rigid synthetic process with graphene-like polycyclic hydrocarbon molecules [69]. Recently, reduced graphene oxide (rGO) has been employed for label-free detection of influenza viruses due to its promising conducting properties and large surface area [70]. For example, the interaction of rGO sulphated derivatives and viruses exhibits the degree of sulfation and polymer density-dependent [71]. The stronger the level of sulfation and the smaller the size, the more powerful effect was observed on herpes virus. It was implied as a combination outcome of the facile bending and shared encapsulation by two or more small GO sheets [71]. In fact, GO scales can wrap up and impound microorganisms by enveloping them in a covering carbon sheet [72]. Interestingly, it has been lately explored on how the SARS-CoV-2 protein-receptor-binding domain could correlate with heparin that in turn alters conformation, which demonstrates how to develop a first-line therapeutic agent against heparin viruses with glycosaminoglycan-based antiviral drugs kin the presence of sulphated derivatives of GO. Graphene is also used for biosensing and bioimaging since photocatalyzed graphene-derived light absorption can be utilized to knock down viral particles. Also, sulfonated magnetic NPs which were functionalized with rGO have been used to effectively track and photothermally diminish herpes simplex virus type 1 (HSV-1) using NIR light [73, 74].
Xiang et al. in 2018 prepared a simple and smart DNA detection probe from GQDs-modified glassy carbon electrode combined with specific sequence DNA molecules to identify hepatitis B Virus (HBV)'s DNA [75]. The probe DNA would adhere to the target, HBV-DNA, instead of GQDs if the target HBV-DNA was found in the test solution. Also, the study suggested that the sensor could be used in detecting other probe DNA with high sensitivity [75].
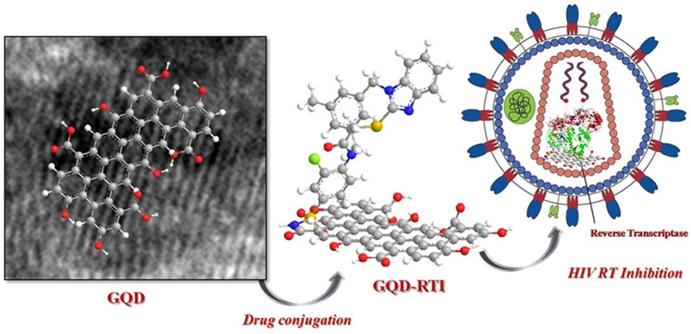
Iannazzo et al. in 2018 reported a type of GQDs prepared from multiwalled carbon nanotubes (MWCNTs) using lengthy acidic oxidation and exfoliation process [76]. The synthesized GQDs were conjugated with the reverse transcriptase inhibitors CHI499 and CDF119 to be retroviral agents as indicated in Figure 11. As a result, the prepared modified GQDs inhibited viral entry of HIV with non-toxicity. Therefore, it is conferred from their promising results that a novel antiviral strategy using GQDs could be explored for HIV inhibition and therapy [76].
(A) The broad-spectrum antiviral efficiency of Gly-CDs. Separate incubation of both Vero and PK-15 cells with Gly-CDs at different concentrations of 0.15, 0.30, 0.60, and 0.90 mg mL-1 for 12 and 24 h. (B) Cellular ROS levels in PPRSV infected MARC-145 cells post different treatments. The cell group shows normal cells not treated by either Gly-CDs or PRRSV. The mock group exhibits the PRRSV-infected cells in the absence of Gly-CDs. The Gly-CDs group indicates the PRRSV-infected cells treated with Gly-CDs. Scale bar = 100 µm. Reprinted with permission from the reference [67].
Carbon dots or other NPs developed for antiviral activities
| CDs | Precursors/Routes | Size, morphology, toxicity, and IC50 | Cells/Virus | Purpose | Reference |
|---|---|---|---|---|---|
| PBA-CDs 3-APBA-CDs 4-APBA-CDs | Boronic acid/ Hydrothermal carbonization | •Size diameters = 332±60 nm, 55 ±1nm, 96 ± 1nm, •Cell proliferation = 67%, 95%, and 97% •IC50 = 80 and 145 mg/mL-1 | •A549 cells & Vero cells/ •HSV-1 | •For stopping viral attachment and entry. | [53] |
| CCM-CDs | Citric acid/ Pyrolysis | •Diameter = 1.5 ± 0.3 nm •Cell proliferation = 90% | •Vero cells/ •Coronavirus | •For inhibiting viral entry •In order to prevent budding of negative-strand RNA in virus. | [77] |
| Young barley leaf-derived B-CDs | Citric acid/ Hydrothermal | •Size in diameter = 1.9 nm •Cell survival ≥ over 85% | •Hela cells & PK-15 cells/ •Pseudorabies virus (PRV) | •For evaluating bioimaging and antiviral effects | [78] |
| Citric acid modified by boronic acid CDs | Carboxyl phenylboronic acid (CBBA)/ Pyrolysis | •Diameter = 2.8 nm - 6.2 nm •Cell proliferation < 80% •IC50 = 9506.3 and 26.7 μg/mL | •MT4 and MOLT-4 cells/ •HIV-1 | •For inhibiting HIV infection | [26] |
| BZM-CDs | Hydrothermal | •Average size = 4.4 ± 0.6 nm •Cell survival rate >80% | •BHK-21 cells & Vero cells/ •Flaviviruses | •For blocking the viral infection | [54] |
| Ethylenediamine + citric acid (CQDs) | Boronic acid/ Hydrothermal carbonization | •Particle size = 6.5 ± 0.2 nm •EC50 = 52 ± 8 μg/ mL | •Huh-7 cells/ •HCoV | •For inhibiting viral replication. | [58] |
| Citric acid and ethylene diamine (CQDs) | Streptavidin/ Hydrothermal | •Particle size = 4 - 5nm | •HIV-1 | •For assessing HIV-1 p24 antigen on improved models | [79] |
| Cur-CQDs | Pyrolysis | •Mean diameter = 4.8 ± 0.8 nm •CC50 = 452.2 µg/ mL •EC50= 0.2 µg/ mL | •RD cells/ •EV71 | •For inhibiting EV71 infection | [59] |
| Spermidine powder -based CDs | Biogenic polyamines/ Pyrolysis | •Size = 6 nm | •Haemolymph of shrimp Litopenaeus vannamei •White spot syndrome virus (WSSV) | For blocking receptor on host cell membrane using recombinant viral proteins or virus antiserum | [80] |
The reverse transcriptase inhibitor-conjugated GQDs as a promising therapeutic candidate for HIV treatment. Reprinted with permission from the reference [76].
The published researches on CDs and other NPs for antiviral activities are summarized in the Table 1.
The collected articles have proven the great efficiency of CD-based antiviral agents derived from molecules, compounds, and combining with complex nano-substances. And promising antiviral results from some published articles of facile prepared-CDs and complex mixed-compounds by surface modifications or conjugation are demonstrated. The above-mentioned articles mostly focused on virus inhibition and the life cycle of viruses affected by the antiviral activity from one or more CD species. In addition, it is clear that CD-based antiviral agents prepared from natural curcumin also exhibited antiviral effects on RNA viruses. Besides, it is noticeable that surface modifications and functionalization of CDs have a highly strong preference in enhancing the efficiency of CD-based antiviral agents.
Photodynamic Inactivation
Photosensitized carbon-based nanomaterials are also needed as sensitive, selective, and affordable biosensors for tracking and killing viruses in this pandemic situation. Henceforth, CDs have been explored in viral as well as bacterial sensing or killing. Biosafety of CDs can influence the diagnosis and management of various viral diseases. The affordable fluorescence feature can make CDs as sensors or photosensitizers for biological as well as non-biological operations [81]. For instance, photodynamic inactivation (PDI) derived from CDs has been used to inactivate microorganisms [82].
Above: A schematic description of CDs on the photoexcited state processes, separating charge, trapping electrons, and creating holes and their radiative recombinations. Below: The energy transformation process with the obtained fluorescence quantum yield (ΦF). Reprinted with permission from the reference [83].
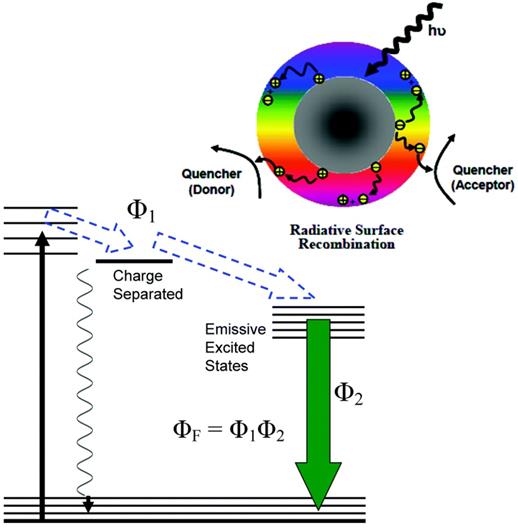
In PDI, a well-known method is to use UV light to illuminate on focused NPs such as colloidal TiO2 to produce ROS, including singlet oxygen, superoxide and hydroxyl radicals, to kill pathogenic bacteria or viruses. Since UV light is harmful for extensive uses in human, TiO2 NPs have been modified to extend absorption to visible length. Upon photoexcitation of CDs, electrons and holes are excited, which are trapped at various defect sites in the dots and might directly take part in the explored antimicrobial activities. Subsequently, the electrons and holes after recombination result in the PL emission of CDs, which also participates in the photodynamic antimicrobial functions [83].
Lim et al. in 2012 presented up-conversion NP-based photodynamic inactivation for potential antiviral uses [84]. Those up-conversion NPs acted as photosensitizers in target biological environment converting NIR to visible emission. Interestingly, the up-conversion NPs significantly reduced the infectious rate of viruses in vitro after photodynamic treatment [84].
Rabe et al. in 2020 designed a type of CDs coupled with oligometric polyethylenimne [83]. The synthesized CDs exhibited visible emission, which readily inactivated multidrug-resistant bacterial strains as depicted in Figure 12 [83]. Highly photosensitized CDs were observed under nature light exposure to inactivate small RNA viruses of MS2 by degrading the viral genomic RNA. However, the prepared CDs showed that no significant damage to the MS2 phage shell [85].
Action and outcome of photoexcited CDs contribute to the PDI capability in highly visible/natural light-activated antibacterial function of CDs. To show more evidences of clear understanding in mechanistic details, CDs need to be further studied for broad applications in the prevention and control of viral infection/spread.
Conclusion and perspectives
In summary, CDs and GQDs were revealed as promising antiviral agents from many studies on HIV or other RNA viruses' inhibition. Various approaches for the preparation of CDs have highlighted their advantages over virus inhibition and treatment in vitro, but a few challenges such as practical testing in vivo systems is still a matter of time. Also, targeting and long-lasting of CDs in virus-infected cells could be significantly enhanced only when their surfaces were coated with diverse ligands. In contrast, although GQDs, GO and graphene-based nanomaterials considerably displayed strong capabilities to detect viruses, their sizes are larger than that of CDs. Subsequently, GQDs are less preferable than CDs since the direct use in vivo could not have controllable and predictable antiviral loading capabilities. In the viewpoint of photodynamic therapy, the responsive polymer materials can be used to prepare NPs, which can respond to enzymes, ROS, or other chemicals abundant in the infection sites to overcome the uncontrolled inflammation and release of cytokines. Nevertheless, it is hoped that aforementioned results encourage the development of CDs and GQDs as antiviral agents for new generations to treat virus infections.
Abbreviations
NPs: nanoparticles; CDs: carbon dots; PL: photoluminescence; PBA: 4-phenylboronic acid; 3-APBA: 3-aminophenylboronic acid; 4-APBA: 4-aminophenylboronic acid hydrochloride; PBMCs: peripheral blood mononuclear cells; CCM-CQDs: cationic curcumin-based carbon quantum dots; BZM-CQDs: benzoxamine-based carbon quantum dots; Cur-CQDs: curcumin-based carbon quantum dots; GO: graphene oxide; GQDs: graphene quantum dots; rGO: reduced graphene oxide.
Acknowledgements
The authors thank Department of Chemistry, Universitas Airlangga, Indonesia and Department of Chemistry, Pakokku University, Myanmar for partial supports in the present study.
ORCIDs
- Yaung Kwee: https://orcid.org/0000-0001-5029-8974;
- Yiqun Zhou: https://orcid.org/0000-0002-6594-9925;
- Mochamad Zakki Fahmi: https://orcid.org/0000-0001-5430-9992.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Sarkar J, Das S, Aich S, Bhattacharyya P, Acharya K. Antiviral potential of nanoparticles for the treatment of Coronavirus infections. J. Trace Elem. Med. Biol. 2022;72:126977
2. Sanjuán R, Nebot MR, Chirico N, Mansky LM, Belshaw R. Viral mutation rates. J. virol. 2010;84:9733-48
3. Steinhauer DA, Holland J. Rapid evolution of RNA viruses. Annu. Rev. Microbiol. 1987;41:409-31
4. Kim PS, Read SW. Nanotechnology and HIV: potential applications for treatment and prevention. Wiley Interdiscip. Rev. Nanomed. 2010;2:693-702
5. Du T, Liang J, Dong N, Liu L, Fang L, Xiao S. et al. Carbon dots as inhibitors of virus by activation of type I interferon response. Carbon. 2016;110:278-85
6. Mamo T, Moseman EA, Kolishetti N, Salvador-Morales C, Shi J, Kuritzkes DR. et al. Emerging nanotechnology approaches for HIV/AIDS treatment and prevention. Nanomedicine. 2010;5:269-85
7. Garg P, Sangam S, Kochhar D, Pahari S, Kar C, Mukherjee M. Exploring the role of triazole functionalized heteroatom co-doped carbon quantum dots against human coronaviruses. Nano today. 2020;35:101001
8. Liu J, Dai S, Wang M, Hu Z, Wang H, Deng F. Virus like particle-based vaccines against emerging infectious disease viruses. Virol. Sin. 2016;31:279-87
9. Mansoori GA, Soelaiman TF. Nanotechnology—an introduction for the standards community. J. ASTM Int. 2005;2:1-22
10. Dacoba TG, Omange RW, Li H, Crecente-Campo J, Luo M, Alonso MJ. Polysaccharide nanoparticles can efficiently modulate the immune response against an HIV peptide antigen. ACS nano. 2019;13:4947-59
11. Kumar A, Ma H, Zhang X, Huang K, Jin S, Liu J. et al. Gold nanoparticles functionalized with therapeutic and targeted peptides for cancer treatment. Biomaterials. 2012;33:1180-9
12. McNeil SE. Unique benefits of nanotechnology to drug delivery and diagnostics. Characterization of nanoparticles intended for drug delivery: Springer. 2011:3-8
13. Singh L, Kruger HG, Maguire GE, Govender T, Parboosing R. The role of nanotechnology in the treatment of viral infections. Ther. Adv. Infect. Dis. 2017;4:105-31
14. Santos-Martinez MJ, Rahme K, Corbalan JJ, Faulkner C, Holmes JD, Tajber L. et al. Pegylation increases platelet biocompatibility of gold nanoparticles. J. Biomed. Nanotech. 2014;10:1004-15
15. Khalil NM, Carraro E, Cótica LF, Mainardes RM. Potential of polymeric nanoparticles in AIDS treatment and prevention. Expert Opin. Drug Deliv. 2011;8:95-112
16. IAO V. Liposomes: from physics to applications; 1993
17. Jimenez JL, Pion M, de la Mata FJ, Gomez R, Munoz E, Leal M. et al. Dendrimers as topical microbicides with activity against HIV. New J. Chem. 2012;36:299-309
18. Shah P, Bhalodia D, Shelat P. Nanoemulsion: a pharmaceutical review. Syst. Rev. Pharm. 2010 1
19. Shegokar R, Jansch M, Singh KK, Müller RH. In vitro protein adsorption studies on nevirapine nanosuspensions for HIV/AIDS chemotherapy. Nanomed.: Nanotechnol. Biol. M. 2011;7:333-40
20. Fasili Z, Mehri F, Ebrahimi HA, Jamali Z, Mohammad Khanlou E, Kahrizi F. et al. Applying nanoparticles in the treatment of viral infections and toxicological considerations. Int. j. pharm. biomed. res. 2019;5:1-20
21. Rai M, Ingle AP, Gupta I, Brandelli A. Bioactivity of noble metal nanoparticles decorated with biopolymers and their application in drug delivery. Int J Pharm. 2015;496:159-72
22. Sharma P, Garg S. Pure drug and polymer based nanotechnologies for the improved solubility, stability, bioavailability and targeting of anti-HIV drugs. Adv. Drug Deliv. Rev. 2010;62:491-502
23. Zhou L, Huang J, Yu B, Liu Y, You T. A novel electrochemiluminescence immunosensor for the analysis of HIV-1 p24 antigen based on P-RGO@ Au@ Ru-SiO2 composite. ACS Appl. Mater. Interfaces. 2015;7:24438-45
24. Chukkapalli V, Oh SJ, Ono A. Opposing mechanisms involving RNA and lipids regulate HIV-1 Gag membrane binding through the highly basic region of the matrix domain. Proc. Natl. Acad. Sci. 2010;107:1600-5
25. Umair M, Javed I, Rehman M, Madni A, Javeed A, Ghafoor A. et al. Nanotoxicity of inert materials: the case of gold, silver and iron. J. Pharm. Pharm. Sci. 2016;19:161-80
26. Fahmi M, Sukmayani W, Khairunisa SQ, Witaningrum A, Indriati D, Matondang M. et al. Design of boronic acid-attributed carbon dots on inhibits HIV-1 entry. RSC Adv. 2016;6:92996-3002
27. Szunerits S, Barras A, Khanal M, Pagneux Q, Boukherroub R. Nanostructures for the inhibition of viral infections. Molecules. 2015;20:14051-81
28. Cojocaru F-D, Botezat D, Gardikiotis I, Uritu C-M, Dodi G, Trandafir L. et al. Nanomaterials designed for antiviral drug delivery transport across biological barriers. Pharmaceutics. 2020;12:171
29. Curley P, Liptrott NJ, Owen A. Advances in nanomedicine drug delivery applications for HIV therapy. Future Sci. OA. 2017;4:FSO230
30. Date AA, Destache CJ. A review of nanotechnological approaches for the prophylaxis of HIV/AIDS. Biomaterials. 2013;34:6202-28
31. Gupta U, Jain NK. Non-polymeric nano-carriers in HIV/AIDS drug delivery and targeting. Adv. Drug Deliv. Rev. 2010;62:478-90
32. Lembo D, Cavalli R. Nanoparticulate delivery systems for antiviral drugs. Antivir. Chem. Chemother. 2010;21:53-70
33. Monroe M, Flexner C, Cui H. Harnessing nanostructured systems for improved treatment and prevention of HIV disease. Bioeng. Transl. Med. 2018;3:102-23
34. Xu X, Ray R, Gu Y, Ploehn HJ, Gearheart L, Raker K. et al. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004;126:12736-7
35. Kwee Y, Kristanti AN, Aminah NS, Fahmi MZ. Design of Catechin-based Carbon Nanodots as Facile Staining Agents of Tumor Cells. Indones. J. Chem. 2020;20:1332-1346
36. Sun Y-P, Zhou B, Lin Y, Wang W, Fernando KS, Pathak P. et al. Quantum-sized carbon dots for bright and colorful photoluminescence. J. Am. Chem. Soc. 2006;128:7756-7
37. Bhaisare ML, Talib A, Khan MS, Pandey S, Wu H-F. Synthesis of fluorescent carbon dots via microwave carbonization of citric acid in presence of tetraoctylammonium ion, and their application to cellular bioimaging. Microchim. Acta. 2015;182:2173-81
38. Kwee Y, Kristanti AN, Siimon K, Aminah NS, Fahmi MZ. Carbon nanodots derived from natural products. S. Afr. J. Chem. 2021;75:40-63
39. Tong T, Hu H, Zhou J, Deng S, Zhang X, Tang W. et al. Glycyrrhizic-Acid-Based Carbon Dots with High Antiviral Activity by Multisite Inhibition Mechanisms. Small. 2020;16:1906206
40. Puvvada N, Kumar BP, Konar S, Kalita H, Mandal M, Pathak A. Synthesis of biocompatible multicolor luminescent carbon dots for bioimaging applications. Sci. Technol. Adv. Mater. 2012;13:045008
41. Yu Y, Li C, Chen C, Huang H, Liang C, Lou Y. et al. Saccharomyces-derived carbon dots for biosensing pH and vitamin B 12. Talanta. 2019;195:117-26
42. He H, Zheng X, Liu S, Zheng M, Xie Z, Wang Y. et al. Diketopyrrolopyrrole-based carbon dots for photodynamic therapy. Nanoscale. 2018;10:10991-8
43. Zhou Y, Zahran EM, Quiroga BA, Perez J, Mintz KJ, Peng Z. et al. Size-dependent photocatalytic activity of carbon dots with surface-state determined photoluminescence. Appl. Catal. b Environ. 2019;248:157-66
44. Oztan CY, Hamawandi B, Zhou Y, Ballikaya S, Toprak MS, Leblanc RM. et al. Thermoelectric performance of Cu2Se doped with rapidly synthesized gel-like carbon dots. J. Alloys Compd. 2021;864:157916
45. Oztan C, Ginzburg E, Akin M, Zhou Y, Leblanc RM, Coverstone V. 3D printed ABS/paraffin hybrid rocket fuels with carbon dots for superior combustion performance. Combust. Flame. 2021;225:428-34
46. Fahmi MZ, Chang J-Y. A facile strategy to enable nanoparticles for simultaneous phase transfer, folate receptor targeting, and cisplatin delivery. RSC Adv. 2014;4:56713-21
47. Dong X, Liang W, Meziani MJ, Sun Y-P, Yang L. Carbon dots as potent antimicrobial agents. Theranostics. 2020;10:671
48. Demirci S, McNally AB, Ayyala RS, Lawson LB, Sahiner N. Synthesis and characterization of nitrogen-doped carbon dots as fluorescent nanoprobes with antimicrobial properties and skin permeability. J. Drug Deliv. Sci. Technol. 2020;59:101889
49. Achadu OJ, Takemura K, Khoris IM, Park EY. Plasmonic/magnetic molybdenum trioxide and graphitic carbon nitride quantum dots-based fluoroimmunosensing system for influenza virus. Sens. Actuators B: Chem. 2020;321:128494
50. Molaei MJ. Carbon quantum dots and their biomedical and therapeutic applications: a review. RSC Adv. 2019;9:6460-81
51. Zhou J, Shan X, Ma J, Gu Y, Qian Z, Chen J. et al. Facile synthesis of P-doped carbon quantum dots with highly efficient photoluminescence. RSC Adv. 2014;4:5465-8
52. Innocenzi P, Stagi L. Carbon-based antiviral nanomaterials: graphene, C-dots, and fullerenes: A perspective. Chem. Sci. 2020;11:6606-22
53. Barras A, Pagneux Q, Sane F, Wang Q, Boukherroub R, Hober D. et al. High efficiency of functional carbon nanodots as entry inhibitors of herpes simplex virus type 1. ACS Appl. Mater. Interfaces. 2016;8:9004-13
54. Huang S, Gu J, Ye J, Fang B, Wan S, Wang C. et al. Benzoxazine monomer derived carbon dots as a broad-spectrum agent to block. J. Colloid. Interface Sci. 2019: 542: 198-206.
55. Aung YY, Kristanti AN, Khairunisa SQ, Nasronudin N, Fahmi MZ. Inactivation of HIV-1 Infection through Integrative Blocking with Amino Phenylboronic Acid Attributed Carbon Dots. ACS Biomater. Sci. Eng. 2020;6:4490-501
56. Gholami MF, Lauster D, Ludwig K, Storm J, Ziem B, Severin N. et al. Functionalized graphene as extracellular matrix mimics: toward well-defined 2D nanomaterials for multivalent virus interactions. Adv. Funct. Mater. 2017;27:1606477
57. Ting D, Dong N, Fang L, Lu J, Bi J, Xiao S. et al. Correction to multisite inhibitors for enteric coronavirus: Antiviral cationic carbon dots based on curcumin. ACS Appl. Nano Mater. 2018;1:5451-9
58. Łoczechin A, Séron K, Barras A, Giovanelli E, Belouzard S, Chen Y-T. et al. Functional carbon quantum dots as medical countermeasures to human coronavirus. ACS Appl. Mater. Interfaces. 2019;11:42964-74
59. Lin CJ, Chang L, Chu HW, Lin HJ, Chang PC, Wang RY. et al. High amplification of the antiviral activity of curcumin through transformation into carbon quantum dots. Small. 2019;15:1902641
60. Xue Y, Liu C, Andrews G, Wang J, Ge Y. Recent advances in carbon quantum dots for virus detection, as well as inhibition and treatment of viral infection. Nano Converg. 2022;9:1-31
61. Khanal M, Barras A, Vausselin T, Fénéant L, Boukherroub R, Siriwardena A. et al. Boronic acid-modified lipid nanocapsules: a novel platform for the highly efficient inhibition of hepatitis C viral entry. Nanoscale. 2015;7:1392-402
62. Ali A, Banerjea AC. Curcumin inhibits HIV-1 by promoting Tat protein degradation. Sci. Rep. 2016;6:1-9
63. Wang M, Tao L, Xu H. Chinese herbal medicines as a source of molecules with anti-enterovirus 71 activity. Chin. Med. 2016;11:1-26
64. Qin Y, Lin L, Chen Y, Wu S, Si X, Wu H. et al. Curcumin inhibits the replication of enterovirus 71 in vitro. Acta Pharm. Sin. B. 2014;4:284-94
65. Dong X, Moyer MM, Yang F, Sun Y-P, Yang L. Carbon dots' antiviral functions against noroviruses. Sci. Rep. 2017;7:1-10
66. Ju E, Li T, Liu Z, da Silva SR, Wei S, Zhang X. et al. Specific Inhibition of Viral MicroRNAs by Carbon Dots-Mediated Delivery of Locked Nucleic Acids for Therapy of Virus-Induced Cancer. ACS nano. 2020;14:476-87
67. Tong T, Hu H, Zhou J, Deng S, Zhang X, Tang W. et al. Glycyrrhizic-acid-based carbon dots with high antiviral activity by multisite inhibition mechanisms. Small. 2020;16:1906206
68. Zhang R, Ding Z. Recent advances in graphene quantum dots as bioimaging probes. J. Anal. Test. 2018;2:45-60
69. Fan Z, Li S, Yuan F, Fan L. Fluorescent graphene quantum dots for biosensing and bioimaging. RSC Adv. 2015;5:19773-89
70. Singh R, Hong S, Jang J. Label-free detection of influenza viruses using a reduced graphene oxide-based electrochemical immunosensor integrated with a microfluidic platform. Sci. Rep. 2017;7:1-11
71. Ziem B, Azab W, Gholami M, Rabe JP, Osterrieder N, Haag R. Size-dependent inhibition of herpesvirus cellular entry by polyvalent nanoarchitectures. Nanoscale. 2017;9:3774-83
72. Palmieri V, Papi M, Conti C, Ciasca G, Maulucci G, De Spirito M. The future development of bacteria fighting medical devices: the role of graphene oxide. Expert Rev. Med. Devices. 2016;13:1013-9
73. Mycroft-West CJ, Su D, Elli S, Li Y, Guimond SE, Miller GJ. et al. The 2019 coronavirus (SARS-CoV-2) surface protein (Spike) S1 Receptor Binding Domain undergoes conformational change upon heparin binding. BioRxiv. 2020
74. Deokar AR, Nagvenkar AP, Kalt I, Shani L, Yeshurun Y, Gedanken A. et al. Graphene-based “hot plate” for the capture and destruction of the herpes simplex virus type 1. Bioconjugate Chem. 2017;28:1115-22
75. Xiang Q, Huang J, Huang H, Mao W, Ye Z. A label-free electrochemical platform for the highly sensitive detection of hepatitis B virus DNA using graphene quantum dots. RSC Adv. 2018;8:1820-5
76. Iannazzo D, Pistone A, Ferro S, De Luca L, Monforte AM, Romeo R. et al. Graphene quantum dots based systems as HIV inhibitors. Bioconjugate Chem. 2018;29:3084-93
77. Ting D, Dong N, Fang L, Lu J, Bi J, Xiao S. et al. Multisite inhibitors for enteric coronavirus: antiviral cationic carbon dots based on curcumin. ACS Appl. Nano Mater. 2018;1:5451-9
78. Liu H, Bai Y, Zhou Y, Feng C, Liu L, Fang L. et al. Blue and cyan fluorescent carbon dots: one-pot synthesis, selective cell imaging and their antiviral activity. RSC Adv. 2017;7:28016-23
79. Kurdekar A, Chunduri LA, Bulagonda EP, Haleyurgirisetty MK, Kamisetti V, Hewlett IK. Comparative performance evaluation of carbon dot-based paper immunoassay on Whatman filter paper and nitrocellulose paper in the detection of HIV infection. Microfluid. Nanofluidics. 2016;20:99
80. Huang H-T, Lin H-J, Huang H-J, Huang C-C, Lin JH-Y, Chen L-L. Synthesis and evaluation of polyamine carbon quantum dots (CQDs) in Litopenaeus vannamei as a therapeutic agent against WSSV. Sci. Rep. 2020;10:1-11
81. Jaleel JA, Pramod K. Artful and multifaceted applications of carbon dot in biomedicine. J. Control Release. 2018;269:302-21
82. Costa L, Faustino M. Neves MGPMS; Cunha Â.; Almeida A. Photodynamic Inactivation of Mammalian Viruses and Bacteriophages.Viruses. 2012;4:1034-74
83. Rabe DIA, Mohammed OO, Dong X, Patel AK, Overton CM, Tang Y. et al. Carbon dots for highly effective photodynamic inactivation of multidrug-resistant bacteria. Adv. Mater. 2020;1:321-5
84. Lim ME, Lee Y-l, Zhang Y, Chu JJH. Photodynamic inactivation of viruses using upconversion nanoparticles. Biomaterials. 2012;33:1912-20
85. Dong X, Edmondson R, Yang F, Tang Y, Wang P, Sun Y-P. et al. Carbon dots for effective photodynamic inactivation of virus. RSC Adv. 2020;10:33944-54
Author Biographies
Dr. Yaung Kwee achieved his B.Sc. (Hons:) in 2010 and M.Sc. in 2012 from the Department of Chemistry, Pakokku University, Myanmar. Also, he recently received his Ph.D. degree (2021), specialized in carbon dots prepared from natural resources under the supervision of Associate Professor Dr. Mochamad Zakki Fahmi and Associate Professor Dr. Alfinda Novi Kristanti at Department of Chemistry, Universitas Airlangga, Indonesia. His research interest is a focus on carbon dots for cancer treatment and HIV-1 inhibition.
Dr. Yiqun Zhou obtained his Post-Doctorate degree in 2021 and also a Research Director at University of Miami, United States. He received his B.S. (2014) in chemistry from Shaanxi University of Science and Technology. And he completed his Ph.D. in chemistry in Prof. Leblanc's group in the University of Miami in the spring semester of 2019. His current research interests include the development of red-emissive carbon dots, printing (text printing and 3D printing), drug delivery using zebra fish as model (transport across the blood-brain barrier, specific bone targeting), thermoelectricity and photocatalysis.
Dr. Mochamad Zakki Fahmi obtained his Ph.D. in Chemical Engineering in 2014 from the National Taiwan University of Science and Technology. Currently, he works as an Associate Professor at the Department of Chemistry, Universitas Airlangga. His focus is on the study of medical purposed nanomaterials used mainly for theranostics of cancer and HIV.
Dr. Madhuri Sharon obtained her PhD at Leicester University, UK and her Post-doc at Bolton Institute of Technology, UK. Presently, she works as a Director for Walchand Centre for Research in Nanotechnology and also a Director for Sharon Institute of Nanotechnology, India. She has many publications of 16 books, 221 articles and 11 patents.
Dr. Alfinda Novi Kristanti obtained her Doctorate in Organic Chemistry from Universite de Haute-Alsace, France, in 1997, under the supervision of Prof. Bernard Muckensturm. At this time, she is an associate professor at the Department of Chemistry, Faculty of Science and Technology, Universitas Airlangga, Indonesia. Her research interest is a focus on Natural Product Chemistry and Organic Synthesis.
![]() Corresponding author: E-mail: s.khanlawngcom.
Corresponding author: E-mail: s.khanlawngcom.

 Global reach, higher impact
Global reach, higher impact