ISSN: 2206-7418
Nanotheranostics 2022; 6(4):451-464. doi:10.7150/ntno.75045 This issue Cite
Research Paper
Comparative effects of free doxorubicin, liposome encapsulated doxorubicin and liposome co-encapsulated alendronate and doxorubicin (PLAD) on the tumor immunologic milieu in a mouse fibrosarcoma model
1. Department of Immunotherapeutics and Biotechnology, Texas Tech University Health Sciences Center School of Pharmacy, Abilene, TX, USA.
2. Oncology Institute, Shaare Zedek Medical Center and Hebrew University-Faculty of Medicine, Jerusalem, Israel.
3. Levco Pharmaceuticals Ltd., Jerusalem, Israel.
4. Department of Pharmacy Practice, Texas Tech University Health Sciences Center School of Pharmacy, Abilene, TX, USA.
Abstract
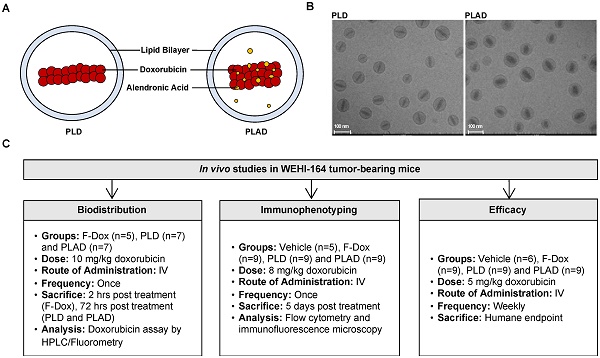
Background: We have previously shown that alendronate, an amino-bisphosphonate, when reformulated in liposomes, can significantly enhance the efficacy of cytotoxic chemotherapies and help remodel the immunosuppressive tumor microenvironment towards an immune-permissive milieu resulting in increased anticancer efficacy. In addition, we have previously shown that the strong metal-chelating properties of alendronate can be exploited for nuclear imaging of liposomal biodistribution. To further improve anticancer efficacy, a pegylated liposome formulation co-encapsulating alendronate and doxorubicin (PLAD) has been developed. In this study, we examined the effects of PLAD on the tumor immunologic milieu in a mouse fibrosarcoma model in which the tumor microenvironment is heavily infiltrated with tumor-associated macrophages (TAM) that are associated with poor prognosis and treatment resistance.
Methods: Doxorubicin biodistribution, characterization of the tumor immunologic milieu, cellular doxorubicin uptake, and tumor growth studies were performed in Balb/c mice bearing subcutaneously implanted WEHI-164 fibrosarcoma cells treated intravenously with PLAD, pegylated liposomal doxorubicin (PLD), free doxorubicin, or vehicle.
Results: PLAD delivery resulted in a high level of tumor doxorubicin that was 20 to 30-fold greater than in free doxorubicin treated mice, and non-significantly higher than in PLD treated mice. PLAD also resulted in increased uptake in spleen and slightly lower plasma levels as compared to PLD. Importantly, our results showed that PLAD, and to a lesser extent PLD, shifted cellular drug uptake to TAM and to monocytic myeloid-derived suppressor cells (MDSC), while there was no drug uptake in neutrophilic MDSC or lymphoid cells. Free doxorubicin cellular drug uptake was below detectable levels. PLAD, and to a lesser extent PLD, also induced significant changes in number and functionality of tumor-infiltrating TAM, MDSC, Treg, NKT, and NK cells that are consistent with enhanced antitumor immune responses in the tumor microenvironment. In contrast, free doxorubicin induced moderate changes in the tumor microenvironment that could promote (decreased Treg) or be detrimental to antitumor immune responses (decreased M1 TAM and NK cells). These immune modulatory effects are reflected in the therapeutic study which showed that PLAD and PLD inhibited tumor growth and significantly prolonged survival, while free doxorubicin showed little or no anticancer activity.
Conclusion: We show that liposomal delivery of doxorubicin not only alters pharmacokinetics, but also dramatically changes the immune modulatory activity of the drug cargo. In addition, our data support that the PLAD nanotheranostic platform further enhances some immune changes that may act in synergy with its cytotoxic chemotherapy effects.
Keywords: chemotherapy, immunotherapy, nanomedicine, bisphosphonate, tumor-associated macrophages, alendronate, doxorubicin, fibrosarcoma

 Global reach, higher impact
Global reach, higher impact