ISSN: 2206-7418
Nanotheranostics 2023; 7(1):70-89. doi:10.7150/ntno.77395 This issue Cite
Review
A systemic review on development of mesoporous nanoparticles as a vehicle for transdermal drug delivery
1. Department of Pharmaceutics and Drug Delivery, School of Pharmacy, University of Mississippi, Oxford, MS - 38677, USA.
2. Department of Pharmaceutical Technology, Faculty of Pharmacy, Kafrelsheikh University, Kafrelsheikh 33516, Egypt.
Abstract
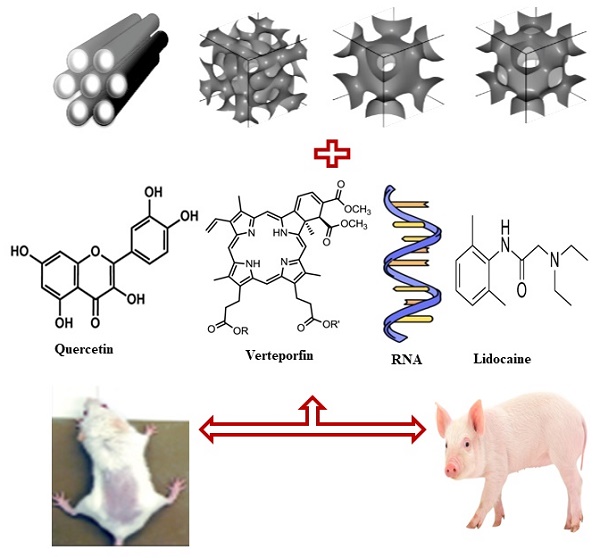
Recent advances in drug delivery technologies utilizing a variety of carriers have resulted in a paradigm shift in the current approach to diagnosis and therapy. Mesoporous silica nanoparticles (MSNs) were developed in response to the need for materials with high thermal, chemical, and mechanical properties. The synthesis, ease of surface functionalization, tunable pore size, large surface area, and biocompatibility of MSNs make them useful in a variety of biomedical applications such as drug delivery, theranostics, and stem cell research. In addition, MSNs have a high capability of delivering actives ranging from small molecules such as drugs and amino acids to larger peptides, vaccines, and antibodies in general. Moreover, MSN-based transdermal delivery has sparked a lot of interest because of the increase in drug stability, permeation, and ease of functionalization. The functionalization of MSNs plays an important role in the efficient delivery of therapeutic agents in a highly controlled manner. This review introduced dermal and transdermal drug delivery systems, explained the anatomy of the skin, and summarized different barriers that affect the transdermal delivery of many therapeutic agents. In addition, the fundamentals of MSNs together with their physicochemical properties, synthesis approaches, raw materials used in their fabrication, and factors affecting their physicochemical properties will be covered. Moreover, the applications of MSNs in dermal and transdermal delivery, the biocompatibility of MSNs in terms of toxicity and safety, and biodistribution will be explained with the help of a detailed literature review. The review is covering the current and future perspectives of MSNs in the pharmaceutical field with therapeutic applications.
Keywords: Mesoporous silica nanoparticles, surface functionalization, tunable pore size, biocompatibility, biodistribution, safety, toxicity

 Global reach, higher impact
Global reach, higher impact