ISSN: 2206-7418
Nanotheranostics 2023; 7(3):258-269. doi:10.7150/ntno.82886 This issue Cite
Review
Current Trends in Nanotheranostics: A Concise Review on Bioimaging and Smart Wearable Technology
Department of Chemical Engineering, National Tsing Hua University, Hsinchu, Taiwan
Received 2023-1-23; Accepted 2023-2-28; Published 2023-3-11
Abstract
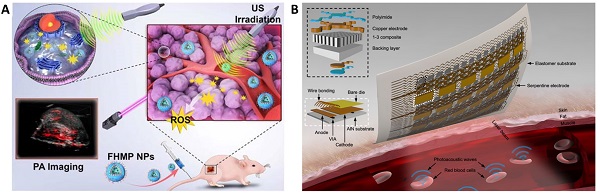
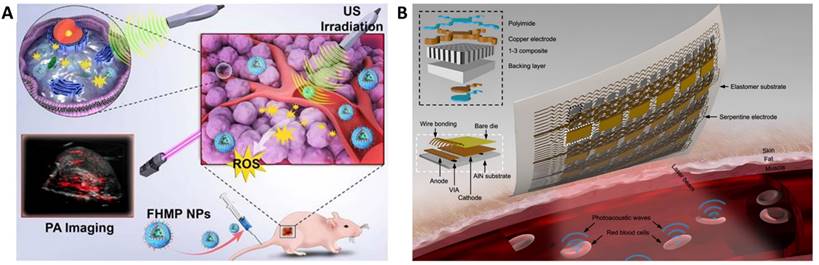
The area of interventional nanotheranostics combines the use of interventional procedures with nanotechnology for the detection and treatment of physiological disorders. Using catheters or endoscopes, for example, interventional techniques make use of minimally invasive approaches to diagnose and treat medical disorders. It is feasible to increase the precision of these approaches and potency by integrating nanotechnology. To visualize and target various parts of the body, such as tumors or obstructed blood veins, one can utilize nanoscale probes or therapeutic delivery systems. Interventional nanotheranostics offers targeted, minimally invasive therapies that can reduce side effects and enhance patient outcomes, and it has the potential to alter the way that many medical illnesses are handled. Clinical enrollment and implementation of such laboratory scale theranostics approach in medical practice is promising for the patients where the user can benefit by tracking its physiological state. This review aims to introduce the most recent advancements in the field of clinical imaging and diagnostic techniques as well as newly developed on-body wearable devices to deliver therapeutics and monitor its due alleviation in the biological milieu.
Introduction
The scope of various therapeutic approaches towards physiological ailments has widened in recent times [1-3]. Conventional therapeutic models such as targeted drug delivery systems are now experiencing a paradigm shift by the technological advancements in disease prognosis and diagnosis [4-6]. The elevated standards of medical technology have brought revolution through smartphones and electronic health that is becoming inclusive to the conventional medical practice [7,8]. Together with smartphones, the convenience of sharing patient health records with the healthcare workers through electronic health platforms is changing the patient treatment experience through remote treatment facilities [9]. Theranostics is such an approach that is focused on therapeutic interventions together with diagnostic tools to facilitate continuous monitoring of the physiological condition in a patient.
Bioimaging
Bioimaging has proved to be an accurate and reliable diagnostic method owing to the visual aid in observing damaged tissue or organ [10]. Technological advancement over the years in in vivo imaging system have overcome the challenges of autofluorescence, low resolution, and closed-circuit camera by improving the penetration depth of the incident laser, that has resulted in non-invasive imaging modalities [11]. In this journey, functional nanomaterials have emerged as an alternative to the conventional organic contrast agents which are known for their detrimental effects on the biological tissues when over exposure [12]. Several different kinds of nanomaterials with fluorescence properties in visible to near-infrared I and II emissions are developed and employed as contrast agents for imaging [13,14]. In this section, we will go through the conventional bioimaging tools that facilitated the visual observation of some of the common physiological conditions followed by the advanced tools with high resolution imaging capabilities. This review will also discuss the applicability of such imaging tools and new contrast agents that have been proposed to use in theranostics and continuous monitoring of the physiological state.
Historically, the application of imaging techniques, that were originally developed for space applications, began in 1960s where researchers explored the possibilities of optical imaging, X-ray imaging and computed tomography (CT), magnetic resonance imaging (MRI) for inspecting food and agricultural products. Further advancements by assembling optical cameras, appropriate illumination and developing computer software began the era of bioimaging that opened a new research area. Currently acceptable clinical imaging techniques such as Positron emission tomography (PET), CT-scan, MRI, ultrasound imaging, or photoacoustic imaging have their own advantages and limitations.
Positron emission tomography (PET)
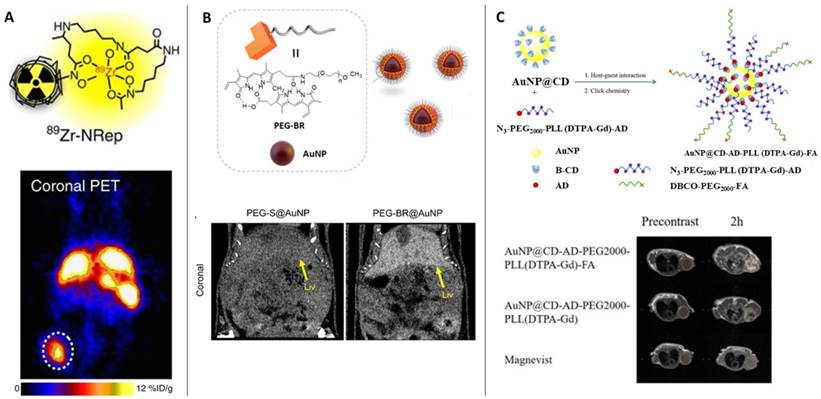
PET is one the most sensitive imaging tools that can reveal functional images at the molecular level with high resolution. Owing to its sensitivity, PET has been employed for various ailments such as cancer, neurodegenerative diseases, coronary artery and assessing myocardial variability. The operation of PET relies on the detection of the gamma rays originating from the annihilation of the positrons emitted from the radioactive molecule introduced in the patient. Among the most popular radioactive molecules, 18F-fluorodeoxyglucose (FDG) is prepared by selectively replacing the oxygen atom by 18F, which is further metabolized by the cells revealing the metabolic processes following the Warburg effect. Similarly, 11C-L-methionine amino acid is used to identify cancer in the biological environment [15]. The radioisotopes used in PET often have long half-life and decay time, thus damaging vital organs of the patient. Recently various forms of 64Cu labelled nanoparticles have been reported as an efficient alternative demonstrating shorter half-life and biocompatibility than the conventional probes [16]. While the artifact signals generated due to the free 64Cu2+ is still a challenge, its doping with other metals prevents aggregation of the probe while extending circulation times and easy excretion. A recent report on the use of 89Zr conjugated Doxil as a nanotheranostic agent was used to assist in imaging the tumor recession and monitor the efficacy of the drugs in PET [17,18] (Figure 1A). The use of radiolabeled probes as PET agents requires functionalization of the drug molecules to the probes to enable dual role [19].
CT-scan
The clinical challenges associated with PET scan such as exposure to radioactive materials and biocompatibility are slightly resolved in computed tomography (CT) scans which are derived from X-ray imaging. Compared to the use of radioactive probes in PET, CT scans functions by collecting and analyzing the cross-sectional X-ray signals that are retrieved from the contrast agents injected in the patient several hours before the test. To obtain high-resolution images, the scan time is longer which necessitates the longer retention and circulation time of the contrast agents. The renal clearance of the contrast agents and prolonged circulation are achieved by controlled size of the contrast agents (larger than the glomerular filtration barrier) and partial diffusion in the blood. The success of the CT scan is justified by its ability to differentiate between soft tissues, water, and bones. Apart from barium sulfate, several different contrast agents based on iodine [20], bismuth [21]; use in picture), gold nanoparticles [22], liposomes [23] have been employed as CT scan contrast agents. Yoo et al showed the fabrication of glutathione-sensitive gold nanoparticles designed by protecting the surface with polyethylene glycol (PEG)-bilirubin (BR) to obtain serum stable contrast agent (Figure 1B). The GSH sensitivity of the contrast agent was exploited to differentiate between healthy liver from a diseased liver through the CT scan [24].
MRI
While PET and CT scans require ionizing radiation for biological imaging, causing serious side effects on human health, magnetic resonance imaging has overcome some inherent problems associated with observing the skeletal and neuronal tissues [25,26]. The use of radiolabeled molecules that respond to the incoming X-ray radiation to generate images of the target organ is often observed to stay longer in the biological system and takes time in clearance from the body. MRI has proved relatively safe owing to the biocompatible contrast agents that is another advantage to high-resolution imaging [27,28]. Conventional contrast agents based on gadolinium are observed to induce acute side effects such as nausea to severe side effects [29], its biocompatibility and efficacy has been improved by its functionalization on gold nanoparticles. A host-guest interaction-based gadolinium assembly on beta-cyclodextrin-modified gold nanoparticles are employed to localize, accumulate, and image tumor region with excellent biocompatibility was reported by Zhang et al [30] (Figure 1C). Also, in a pilot clinical trial, sentinel lymph node melanoma was imaged through the superparamagnetic iron oxide to overcome the short-half-life of the radiolabeled probe 99mTechnetium, which requires single photon emission computed tomography (SPECT-CT) and a tedious standard operating procedure [31]. Despite the inherent challenge to apply MRI as theranostic agents, a significant contribution aiding to its applicability is reported based on the response of the contrast agents against pH, temperature, enzyme, redox reactions, ultrasound, and near infrared light [32].
While these imaging modalities are currently used in clinical practice, the demand for efficient and biofriendly contrast agents with ease of handling is growing. Due to the rise in electronic health and precision therapy, theranostics tools capable of monitoring physiological conditions are gaining popularity. Several innovative researches have brought diagnosis and therapy together with patient-controlled delivery of therapeutic agents, thereby facilitating personalized medication. The following section shows the advancements in the direction of theranostics and patient compliance.
Ultrasound and photoacoustic imaging
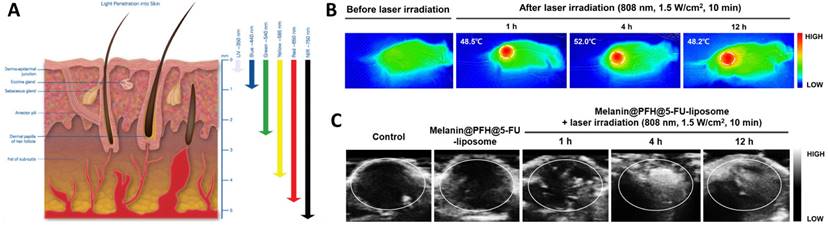
As highlighted above, the clinical application of probe-based imaging tools poses a health risk due to their radioactivity and biocompatibility. On the contrary, light and sound waves are relatively much safer alternatives to gather information from biological system. The spectrum of light encompassing UV, Vis, NIR, IR, are known to penetrate under the skin and reach deeper tissues and organs (Figure 2A) [33]. Sound waves on the other hand due to their longer wavelength, can penetrate deep tissues easily without any significant optical obstruction due to skin or microscopic cellular environment [34]. Supplemented with appropriate contrast agents, ultrasound imaging generates high-contrast images comparable to other imaging tools [35]. Owing to its interaction and absorption by the biological tissues, ultrasound can also cause temperature rise, resulting in activation of the immune response or cellular death due to ablation [36,37]. This phenomenon is widely explored in designing sonodynamic therapy for cancer and non-invasive glucose regulation for treating diabetes [38,39]. In a recent study, the peripheral-focused ultrasound method was demonstrated to stimulate the hepatoportal nerve plexus to alter glucose concentrations in the blood. This method was tested on several animal models for chronic stimulation that can reverse the onset of hyperglycemia by targeting the liver-brain pathway [39]. Ultrasound irradiation causes the production of cavitation-based microbubbles in the biological environment which is explored in ultrasound-assisted bond cleavage for releasing drugs from liposomes [40] and inorganic nanoparticles [41].
(Column A) The liposomal nanoreporter 89Zr-NRep modified with 89Zr-chelating desferrioxamine (DFO) used as PET contrast agent; Representative images of 4T1 tumour-bearing mice with high 89Zr-NRep uptake [3]. Copyright 2016, Nature. (Column B) Schematic illustration of the fabrication of polyethylene glycol (PEG)-bilirubin (BR) protected gold nanopartilces (AuNPs) as glutathione-sensitive CT contrast agent; Representative images of the abdomens of healthy mice administered PEG-BR@AuNPs compared to thiolated PEG-S@AuNPs shows bright contrast with PEG-BR@AuNPs in the liver [24]. Copyright 2021, American Chemical Society. (Column C) Schematic representation of the multifunctional AuNP-based MRI contrast agent protected by cyclodextrin, 1-adamantanecarbonyl chloride, polyethylene glycol, lysine and diethylenetriaminepentacetate acid bound gadolinium conjugated with folic acid (AuNP@CD-AD-PEG2000-PLL(DTPA-Gd)- FA); Comparison of magnetic resonance images of tumor-bearing mice injected with different contrast agents (yellow dot: tumor region) [30]. Copyright 2022, Elsevier.
(A) Illustration showing the depth to which human skin is penetrated by light. While blue and ultraviolet light scarcely enter tissue, red light extinguishes at 4-5 mm beneath the skin's surface [33] Copyright 2017, Springer. (B) IR thermal images showing Melanin, Perfluorohexane (PFH) and 5-fluorouracil (5-FU) loaded liposomes (melanin@PFH@5-FU-liposomes) generating heat upon NIR 808 nm light. (C) Ultrasound images of tumor tissues in CT26-bearing mice before and after laser irradiation at 1 h, 4 h, and 12 h after intravenous injection of melanin@PFH@5-FU-liposomes [40]. Copyright 2022, Springer Nature.
Various sonosensitizers producing reactive oxygen species in an aqueous environment have been employed to treat cancer [42]. Inorganic nanoparticles such as TiO2 and Zr-MOFs are excellent sonosensitizers; their efficiency has been improved by introducing other transition metal groups like Fe3O4 to form Janus nanostructure [43] or creating oxygen-deficient surfaces [44]. TiO2 is popularly known as a sonosensitizer; however, it suffers from low transduction efficiency due to the rapid recombination of the electron-hole pairs. Owing to its biocompatibility, improving the sonodynamic transduction efficiency to produce ROS will be promising for cancer therapy and theranostics applications. The coating by Fe3O2 on TiO2 improves the transduction efficiency by narrowing the bandgap of TiO2, thereby reducing the recombination rate. Similarly, ZrO2-deficient nanoparticles showed higher efficiency in producing ROS under the US due to the more significant separation of the electron-hole pairs. The biocompatibility of ZrO2-x nanoparticles was improved by capping with amine-polyethylene glycol with high absorption in the NIR-II window. It is seen that the NIR-II irradiation also triggers ROS generation in the biological tissue, which is an intriguing approach to employing NIR-II-based therapeutic activation and imaging. Despite the ease of operability of ultrasound imaging in observing SDT activity [40], the technique primarily suffers from low resolution and interference from surrounding tissues (Figure 2B and 2C). Technological advancements such as bio adhesive ultrasound are intriguing approach for on-body continuous organ monitoring [45]. The device is made of a rigid ultrasound probe coupled in-between a soft hydrogel elastomer hybrid, that can transmit high acoustic waves and prevent its detachment from the skin. The bio adhesive ultrasound probe could adhere on the skin and stays without deformation for over 48 hours.
Molecular imaging is another upgrade in medical imaging providing information about the biochemical processes in the biological milieu. The resulting images overcome the limitations of poor spatial resolution and radiolabeling in other imaging tools. Based on its sensitivity towards nanocontrast agents, photoacoustic imaging has been employed in molecular imaging for several applications. J-aggregates of organic dyes are known to exhibit emission in longer wavelengths that further translate to photoacoustic imaging contrast agents. Indocyanine green (ICG) are also shown as a potential contrast agent due to its stable J-aggregates which are encapsulated in a liposome for high sensitivity and estimating saturated oxygen in blood vessels [48]. Other contrast agents such as gold [49,50], silver [51], copper [52], zinc selenide [53], iron [54], cyanine dyes [55] are employed in imaging brain related pathological conditions [56], tumor volumes [57], CAR-T cells [58].
The success of photoacoustic imaging for diagnosis still relies largely on the efficiency contrast agents. However, the need for high intensity laser light to facilitate skin penetration may lead to low resolution due to optical attenuation. Other factors such as targeted tissue localization and ultrasound obstruction through gas cavities or lung tissues affect the resolution of the image [59]. Addressing its sensitivity towards optical absorption and ultrasound generation, Gao et al developed a wearable continuous monitoring epidermal patch. The vertical-cavity surface-emitting laser diodes (VCSEL) arranged in an array on the epidermal patch can generate laser pulses to penetrate the skin and a piezoelectric transducer array for detecting the resulting ultrasound waves. The hemoglobin in the blood expands due to the localized heating under incident laser thus causing an acoustic wave which is detected by the piezoelectric transducer. Such an epidermal patch can generate high spatial resolution image of single molecules (Figure 3B) [60].
(A) Schematic illustration of the multifunctional nanoplatform for efficiently photoacoustic imaging-guided sonodynamic therapy to tumor cells/tissue [47]. Copyright 2018, IvySpring. (B) Schematics of soft-photoacoustic patch for detection of hemoglobin and measuring temperature. The hemoglobin molecules in red blood cells go through a thermoelastic expansion after absorbing optical energy, which then transmits acoustic waves into the surrounding media. The transducer array will gather the photoacoustic waves and then transmit them to a backend system for data processing [60]. Copyright 2022, Nature.
Light-based theranostics
Light based imaging modalities based on fluorescent nanomaterials are another approach to visualize biological processes and structures in living cells and tissues [61,62]. These materials have unique optical and physical properties that make them ideal for bioimaging applications, such as high sensitivity, selectivity, and the ability to be functionalized with specific biomolecules. Examples of nanomaterials used in fluorescence bioimaging include quantum dots [63], gold nanoparticles [64-66], and fluorescent dyes [67]. Quantum dots, which are also semiconductor nanocrystals, have particularly high fluorescence efficiency and broad absorption spectra, making them useful for multiplexing and deep tissue imaging [68,69]. Gold nanoparticles have strong light scattering properties and can be used to enhance the signal of fluorescent probes [70,71], while fluorescent dyes can be used to label specific biomolecules or structures [72]. The use of nanomaterials in fluorescence bioimaging is expected to have a significant impact on the early diagnosis and treatment of diseases, as well as on our understanding of biological processes at the molecular level.
Fluorescent nanomaterials have been at the forefront of the bioimaging-based tracking of biological tissues. Various functional groups have been reported to facilitate the functionalization of nanomaterials, acting as a probe for a visual aid of the targeted tissue. Targeting adipose tissues is a significant challenge due to their widespread availability as a protective layer surrounding vital organs and metabolic activity. Brown adipose tissue, among the other types, viz. Beige and White adipose tissue, actively metabolize fatty acids when subjected to low temperatures. Owing to the inherent fatty acid composition, the adipose tissue is non-responsive to alternate detection methods such as electrical signals, thus, leaving an optical or ultrasound-based approach [73]. Taking advantage of the fatty acid metabolism and its inherent affinity towards adipose tissue, Paulus et al. reported the applicability of a BODIPY functionalized fatty acid tracer for PET and fluorescence imaging [74]. The in-situ esterification of the surface fatty acid to triglyceride, followed by its entrapment into chylomicron-like particles, assists its absorption by the intestines and traverses through the lymphatic system to the brown adipose tissues. When subjected to varying temperatures to instigate fatty acid metabolism in the brown adipose tissue, the BODIPY dye is activated, resulting in sub-cellular imaging of the adipose tissue. A decade of research on the progression of cancer is now correlated to the surrounding adipose tissue, primarily brown adipose tissue [75,76]. Hypoxia instigating therapeutics and radiotherapy have made cancer cells strive for fatty acid metabolism pathway for survival through molecular signaling. The adipose tissue surrounding tumor undergoes fatty acid metabolism and produces energy through TCA cycle, that causes the patient with lean body and loss in weight due to excess fatty acid metabolism. Taking advantage of the surrounding adipose tissue, Wen et al proposed a formulation composed of rumenic acid, doxorubicin prodrug, fatty acid binding protein conjugated at a radical sensitive chemical bond, which is loaded on adipose cells and locally delivered at tumor site [77]. Owing the fatty acid binding protein and rumenic acid, the formulation can be stored in the adipose cells, and H2O2 catalyzed separation of the prodrug causes tumor cell death, resulting in highly efficient therapy. Conjugating fluorescent probe on similar designs could facilitate a theranostics solution to monitor the treatment progress of the tumor.
Using optogenetics, researchers have developed methods to release therapeutic genes using visible light from smartphone as a convenient source to activate the release process, with the goal of making treatment more patient friendly. A recent study by Mansouri et al [78] has shown that the green light from smartwatch is able to remotely control transgene activation and demonstrate the release of human glucagon-like peptide-1 from engineered human cells to treat diabetes. Such an approach is highly promising as the current available smartwatches can project green and red light (corresponding to 540 nm and 690 nm), which is biofriendly and could be used to design various theranostics approaches.
Current fluorescence-based bioimaging modalities employing nanomaterials as contrast agents suffer from certain limitations such as altered physicochemical properties post-interaction with the biological entities in situ, photo instability of the probes, long duration of the imaging, photon scattering and tissue absorption at the visible or near infrared region of the spectrum with poor spatiotemporal resolution [79,80]. To overcome these challenges, nanomaterials capable of absorbing in the NIR-II region of the spectrum, with high emission intensities are developed using lanthanides, carbon nanotubes, quantum dots, and organic-inorganic complex. The tunable biocompatibility of the next generation NIR-II probes enables its applicability as a stimuli-responsive therapeutic delivery vehicle as well as an efficient probe to the biochemical reactions [81-83]. Owing to its facile electron energy transfer in the NIR-II region, multiplex layers of lanthanide elements are explored in the field of bioimaging producing high resolution images of blood vessels in animal models [84,85].
Nanotechnology and theranostics
Wearable devices are being developed for the therapeutic treatment of various organs and systems in the body. For example, wearable devices are being used to deliver insulin to patients with diabetes [86], to provide pain management for individuals with chronic pain conditions [87], and to deliver targeted chemotherapy to cancer patients [88,89]. Wearable devices are also being developed for the treatment of cardiovascular diseases, such as by delivering medications or providing electrical stimulation to the heart [90-92]. In the field of neurology, wearable devices are being used to deliver brain stimulation to treat conditions such as Parkinson's disease and depression [93,94]. These devices have the potential to improve patient compliance and outcomes, as they allow for continuous or intermittent therapy while the patient goes about their daily activities. Wearable therapeutics are an emerging field with great potential to revolutionize the way that many medical conditions are treated. In this section we will see the current trends in the integration of smartphone technology and electronic health in theranostics.
Wound healing
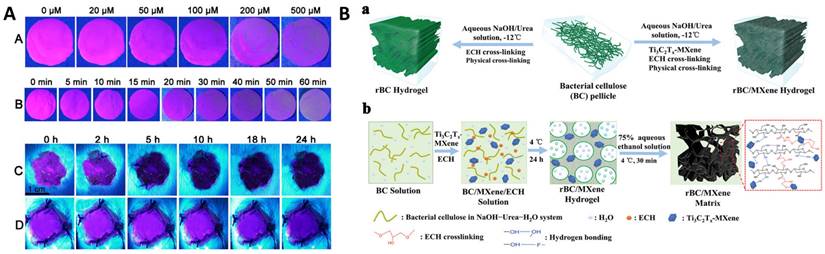
The biomolecular reactions occurring at the wound site during the wound healing process are categorized into four stages, viz. hemostasis, inflammation, proliferation, and remodeling. While hemostasis lasts a few minutes post injury, inflammation and proliferation are the determinants of the wound status and its healing capabilities which lasts from 5 to 20 days. In the early two stages of wound healing, blood clotting is followed by platelets degranulation at wound site promotes the release of a series of mediators from basophils and mast cells causing vasodilation, which further instigates the secretions of fibrinogen and other chemoattractant resulting in swelling of the wound. During proliferation, tissue granulation and angiogenesis begin to contract the wound area with the secretion of several growth factors while in the remodeling stage, the endothelial cells along with macrophages remodel the wound area by replacing damaged cells through apoptosis [95]. On the contrary to normal wound, chronic wound is among serious terminal diseases due to impaired healing process, which is characterized by complications in wound debridement and biofilm formation due to wound dressings. Current wound management is primarily focused on regular debridement and reapplication of absorbable or non-absorbable dressing based on the site of the wound. Conventional wound dressings made of fabric loaded with antibiotic and stimuli responsive biomolecules [96] are now being replaced by biological alternatives such as fish scales, organic hydrogel [97], DNA hydrogels [98], organic and inorganic nanomaterial-hydrogel composite dressings [99,100], oxygenated wound site [101] through oxygen releasing microspheres loaded in polymer matrix [102] and other approach [103-105]. Among the plethora of biomarkers secreted in the wound exudate [106], dynamic concentrations of H2O2 are associated with the healing stages [107,108]. The concentration of H2O2 is significantly high in the hemostasis and inflammation stage while gradually reducing through the proliferation and remodeling stages. Owing to its dynamic localized concentrations, H2O2 has attracted the development of smart composite dressings that can exhibit functional properties in the form of fluorescence or electrical signals. Wu et al developed europium coordinated polymers loaded into polyacrylonitrile nanofiber mats which can absorb the wound exudate and exhibit varying fluorescence intensity after interaction with localized H2O2 concentration (Figure 4A) [109]. Moisture retention, absorption of the wound exudate and antibiotic are necessary factors to promote healing of chronic wounds and prevent biofouling. Wound debridement and periodic change of dressing are deemed necessary to aid in rapid healing. Promoting cellular migration of endothelial cells and macrophage to the wound site assists in the proliferation and remodeling stages. It is seen that electrical stimulation over the epidermis could attract these cells to the wound site and promote rapid healing [110-112]. To facilitate electrical stimulation through wound dressing, conducting polymers (such as PEDOT:PSS [113], PAni [114]), triboelectric nanogenerators [115,116] are incorporated into hydrogels. The composite dressings are integrated with electrical signal measuring tools and bridged to smartphone for continuous monitoring of the healing progress [117,118]. MXenes are recent exploration in this direction of electroactive wound dressing, embedded in bacterial cellulose to promote cellular proliferation and migration at the wound site [119,120]. In a typical fabrication, as shown in Figure 4B (a), the bacterial cellulose (BC) pellicles were resolved using NaOH and epichlorohydrin (ECH) cross linker to obtain regenerated bacterial cellulose (rBC). The physical and chemical cross linking of Ti3C2Tx MXene into the BC solution in the presence of ECH resulted in rBC/MXene matrix conducting hydrogel (Figure 4B (b)). In another approach, Kalidasan et al have demonstrated a wirelessly controlled sutures made of PEDOT:PSS conducting polymer which operates at 1-2 GHz to monitor deep surgical wounds [121].
(Panel A) Fluorescent color change in Polyacrylonitrile (PAN)-europium (Eu) coordination polymer mats at excitation wavelength 397 nm: (A) with different concentration of H2O2, (B) with different incubation time of H2O2 (200 μM); (C) on the wound after bacterial non-infection and (D) on the wound after bacterial infection [109]. Copyright 2020, Elsevier. (Panel B) (a) Schematics representation of the fabrication of regenerated Bacterial Cellulose (rBC)-based hydrogels incorporating Ti3C2Tx MXene through chemical and physical crosslinking; (b) Diagram of the mechanism of synthesizing rBC/MXene composite hydrogels [119]. Copyright 2020, Wiley.
Vagus nerve
The vagus nerve is a long, complex nerve that extends from the brainstem to the abdomen and controls a wide range of functions, including heart rate, digestion, and the immune system [122]. Monitoring the vagus nerve can provide important information about the body's physiological state and can be used to diagnose and treat a variety of medical conditions. For example, vagus nerve stimulation (VNS) has been used to treat epilepsy [123], depression[124], and other conditions [125] by delivering electrical impulses to the nerve. In addition, the vagus nerve is involved in the body's response to inflammation and monitoring the vagus nerve can provide insights into the immune system and the development of inflammatory conditions [126,127]. The vagus nerve is also a key component of the parasympathetic nervous system, which helps to regulate rest and relaxation, and monitoring the vagus nerve can provide insights into stress and other psychological factors [122]. Overall, the vagus nerve is a critical part of the body's overall functioning and monitoring it can provide important information for the diagnosis and treatment of a wide range of medical conditions.
Due to its association with Parkinson's disease, epilepsy, clinical depression, and gastroparesis, biomedical engineers are interested in tapping the potential of vagus nerve to address these challenges. The current clinical implant is placed at the lower neck to stimulate and monitor the vagus nerve in a critical surgical procedure. Alternatively, transcutaneous vagus nerve stimulation is at the forefront in clinical trials as an effective non-invasive approach [128-130]. Another alternative to battery powered metallic implants is based on triboelectric nanogenerators. Graphene-loaded polyacrylamide hydrogel triboelectric nanogenerator (HENG) [131] were shown to generate alternating electric current of 1.6 mA after exposed to 0.3 W/cm2 focused ultrasound through vibration induced compression in the electric double layer of the hydrogel. Due to its self-powered battery-free and biocompatible property, such technology holds great promises to remote controlled vagus nerve stimulation and monitoring. The innervation of the vagus nerve in the visceral adipose tissue and the gut region makes it an attractive therapeutic approach to alleviate the psychological and physiological condition of the patient [130,132,133].
Obesity
While it is well known that adipose cells tend to exist in the body for long periods, slow rate of triglyceride removal against its gradual buildup over the years is major cause of obesity [134]. In recent years, obesity has turned to be a major public health problem that is associated with a range of negative health outcomes, including diabetes, heart disease, and stroke. Adipose tissue, or fat, is a key factor in the development of obesity, and imaging techniques can be used to visualize and measure adipose tissue in the body. There are a number of imaging techniques that can be used to assess adipose tissue, including computed tomography (CT), magnetic resonance imaging (MRI), and ultrasound [135]. CT and MRI can provide detailed images of fat tissue and allow for the measurement of fat volume and distribution. Ultrasound can also be used to measure fat thickness and to evaluate the structure of fat tissue. In addition, newer techniques such as positron emission tomography (PET) and single photon emission computed tomography (SPECT) can be used to measure metabolism and blood flow in adipose tissue. These imaging techniques can provide important information about the distribution and function of fat tissue and can be used to evaluate the effectiveness of weight loss interventions [133].
Despite various approaches to the treatment of obesity, there is still room to develop an effective theranostics approach demonstrating effective drug delivery and simultaneous detection adipocyte shrinkage [136].
Depression and mental health
In recent years, chronic stress and subsequent depression is a growing concern of modern society affecting a large population across the globe. The severity is many folds when its identification is highly subjective and where attempts to correlate the levels of chemical signaling molecules in the blood with the clinical diagnosis for depression are on the rise [137]. Cortisol, a steroid hormone is an active biomarker for physiological as well as psychological stress [138]. High levels of cortisol suggest chronic stress with signs of anxiety and depression along with altered blood pressure, blood glucose and a plethora of physiological ailments. Clinical therapeutics in the form of suppressants pose a threat of addiction and severe damage to vital organs. Thus, it is imperative to keep track of stress hormones, or the therapeutics administered to regulate the levels of cortisol. Contrast to point-of-care biosensors, wearable devices and micro patches are gaining immense popularity due to its real-time information and overcoming the artifacts in fluorescence or optical sensors methods. Parlak et al have shown the fabrication of wearable electrochemical device for identifying cortisol from sweat [139]. Conductive polymers, such as PEDOT:PSS, come handy for the fabrication of on-body wearable patches for sweat-based biomarker detection. For the fabrication of the on-body cortisol sensor, organic electrochemical transistor composed of PEDOT:PSS was prepared with an electrolyte solution for gating. The OECT would transduce the cortisol ions entering into the sensor into electrical signal through molecularly imprinted polymers as receptors to the neutral charge cortisol. Tang et al demonstrated the applicability of a Prussian blue oxidation-based on-body stretchable sensor for cortisol [140]. The sensor was prepared using polypyrrole deposition on molecularly imprinted polymer electrodes in presence of cortisol along with Prussian blue dye, followed by elution of cortisol from the membrane. The elution of cortisol from the membrane creates a cavity resulting in current flowing through the Prussian blue dye. When cortisol from sweat is absorbed back to the cavity, the oxidation current flowing through the dye in polypyrrole membrane is reduced, thus correlated to the cortisol levels in the sweat. The scope of applicability of micro patches or sensors to monitor biomarkers from sweat is widening and offers great promises to integrate with smartphones or other electronic health devices.
Electroceuticals
Electroceutical are categorized as therapeutics targeting neural circuits of the internal organs [141]. Treatment examples include vagus nerve stimulation [142], spinal cord stimulation [143], cochlear [143] and retinal [144] stimulation through biocompatible implants. This approach is extended in targeting chronic lung disorders [145], hypertension [146], diabetes and gastrointestinal diseases [147]. Implants based electrical stimulation have been proved effective to certain diseases such as cardiovascular pacemakers, the next generation of electroceuticals are more targeted towards self-powered and biodegradable implants. As cited previously, hydrogel nanogenerators are developed for vagus nerve stimulation [148]. The innervation of pain receptors in the adipose tissue underlying the subcutaneous opens new avenues for non-invasive detection and stimulation of these electrical signals that will provide information about the user's physiological condition [149]. Among the other methods to read the electrical signals such as voltage, resistance, current or tissue penetrating light through a wearable device, impedance is based devices are relatively easy to calibrate for measuring subcutaneous or deep tissue electrical signals [150]. Nanomaterials and injectable composite hydrogels for localized electrical stimulation coupled with bioimpedance measurements could prove an effective approach for therapeutic delivery and continuous monitoring of the disease condition.
On-body wearable monitoring systems
Wearable devices have the potential to revolutionize the way that physiological challenges such as cancer are monitored and treated. These devices can continuously monitor a patient's vital signs and other health parameters, such as temperature, oxygen saturation, and heart rate. Wearable devices can also be used to deliver targeted therapy to cancer patients, such as by releasing chemotherapy drugs directly to the site of cancer [151,152]. This can help to minimize side effects and improve patient outcomes. In addition, wearable devices can be used to monitor the effectiveness of cancer treatments, such as by measuring the size of tumors over time. Overall, wearable devices have the potential to improve cancer care by providing real-time monitoring and treatment and by enabling personalized medicine approaches. A pioneering effort towards continuous monitoring of tumor volume was presented by Abramson et al [153]. A thin elastomeric film was developed as a conformal, wearable strain sensor that, when placed on the skin of a mouse-bearing subcutaneous tumor, would deform from the original strain due to the corresponding volume of the tumor. The working principle of the strain sensor relies on the change in electrical resistance of the drop-casted 50 nm gold layer on top of the elastomer. As the tumor volume changes in dimensions, the electrical resistance in the gold layer is changed. Owing to the percolation network formed in the elastomer, the resistance in the sensor changes exponentially, corresponding to the tumor volume. This method is up-and-coming in the direction of tracking the biological activity in the skin and potentially translates to other epidermal challenges.
Several new approaches in the direction of nano theranostics in lung cancer are highlighted in this review [154]. Cuffless measurement of blood pressure is a new challenge in wearable devices that would enable the integration of the method into smartphones and smartwatches for a more compliant and quick way to keep track of cardiovascular health. Bioimpedance is a viable approach capable of identifying electrical signals from deep tissues, which is a significant advancement to other methods based on optical, acoustic or pressure sensors. Popular smartwatches available to the general population can detect heart rate but are inefficient to detect blood pressure due to limited penetration depth and signal retention from deep tissue. Kireev et al have addressed this problem by designing a cuffless monitoring system for arterial blood pressure using graphene tattoos through bioimpedance [155].
Outlook and Conclusion
Clinical imaging techniques are powerful tools that are used to visualize and diagnose a wide range of medical conditions. However, these techniques also have certain limitations that need to be considered. For example, many clinical imaging techniques involve the use of ionizing radiation, which can be harmful to the patient if used excessively. Some imaging techniques, such as computed tomography (CT) and nuclear medicine, are particularly high in radiation dose, and care must be taken to ensure that the benefits of the examination outweigh the risks [156]. Other limitations of clinical imaging techniques include their cost, which may not be accessible to all patients, and their availability, as some specialized equipment may only be found at large medical centers. In addition, some imaging techniques, such as magnetic resonance imaging (MRI), are contraindicated for patients with certain medical implants, such as pacemakers. Finally, the interpretation of imaging studies requires specialized training and expertise, and incorrect interpretation can lead to misdiagnosis and inappropriate treatment.
For cancer theranostics and obesity, it is now shown that the Brown adipose tissue is closely related to cancer progression and overcoming hypoxia-based treatment hindrance in the tissue. BODIPY-based fluorescent nanoprobe have been shown to target the adipose tissue, primarily the brown adipose tissue, that opens new avenues towards cancer theranostics. Nanomaterials with properties of targeting the adipose tissue and exhibiting fluorescent response upon excitation under NIR-II laser could be a new direction to tackle cancer. Photoacoustic imaging is another promising tool to observe and narrow down the field of vision to blood cells and single cells. Coupled with ultrasound imaging or electrical impedance, such methods can be useful for monitoring the treatment progress. Interventional nanotheranostics encompassing both therapeutics and diagnosis/monitoring of the treatment progress is a promising and enticing approach that would aim at patient compliant and patient friendly treatment.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Ai X, Mu J, Xing B. Recent Advances of Light-Mediated Theranostics. Theranostics. 2016;6:2439-57
2. Wu Y, Vazquez-Prada KX, Liu Y, Whittaker AK, Zhang R, Ta HT. Recent Advances in the Development of Theranostic Nanoparticles for Cardiovascular Diseases. Nanotheranostics. 2021;5:499-514
3. Luo N, Li J, Dong R, Lu J. Exosome-Based Theranostics for Liver Diseases. Dis Markers. 2022;2022:1-5
4. Kevadiya BD, Ottemann BM, Thomas M ben. et al. Neurotheranostics as personalized medicines. Adv Drug Deliv Rev. 2019;148:252-89
5. Xu M, Zhang K, Song J. Targeted Therapy in Cardiovascular Disease: A Precision Therapy Era. Front Pharmacol. 2021 12
6. Abdelsayed M, Kort EJ, Jovinge S, Mercola M. Repurposing drugs to treat cardiovascular disease in the era of precision medicine. Nat Rev Cardiol. 2022;19:751-64
7. Majumder S, Deen MJ. Smartphone Sensors for Health Monitoring and Diagnosis. Sensors. 2019;19:2164
8. Smartphone Based Medical Diagnostics. Elsevier; 2020
9. Reddy LKV, Madithati P, Narapureddy BR. et al. Perception about Health Applications (Apps) in Smartphones towards Telemedicine during COVID-19: A Cross-Sectional Study. J Pers Med. 2022;12:1920
10. Qi J, Hu X, Dong X. et al. Towards more accurate bioimaging of drug nanocarriers: turning aggregation-caused quenching into a useful tool. Adv Drug Deliv Rev. 2019;143:206-25
11. Weissleder R, Nahrendorf M. Advancing biomedical imaging. Proceedings of the National Academy of Sciences. 2015;112:14424-8
12. Mettenbrink EM, Yang W, Wilhelm S. Bioimaging with Upconversion Nanoparticles. Adv Photonics Res. 2022;3:2200098
13. Zhao J, Zhong D, Zhou S. NIR-I-to-NIR-II fluorescent nanomaterials for biomedical imaging and cancer therapy. J Mater Chem B. 2018;6:349-65
14. Kim D, Jeong K, Kwon JE. et al. Dual-color fluorescent nanoparticles showing perfect color-specific photoswitching for bioimaging and super-resolution microscopy. Nat Commun. 2019;10:3089
15. Jiang Chalich, Deen. Sensors for Positron Emission Tomography Applications. Sensors. 2019;19:5019
16. Chen X, Niu W, Du Z, Zhang Y, Su D, Gao X. 64Cu radiolabeled nanomaterials for positron emission tomography (PET) imaging. Chinese Chemical Letters. 2022;33:3349-60
17. Pérez-Medina C, Abdel-Atti D, Tang J. et al. Nanoreporter PET predicts the efficacy of anti-cancer nanotherapy. Nat Commun. 2016;7:11838
18. Goel S, England CG, Chen F, Cai W. Positron emission tomography and nanotechnology: A dynamic duo for cancer theranostics. Adv Drug Deliv Rev. 2017;113:157-76
19. Pérez-Medina C, Abdel-Atti D, Tang J. et al. Nanoreporter PET predicts the efficacy of anti-cancer nanotherapy. Nat Commun. 2016;7:11838
20. Zhang P, Ma X, Guo R. et al. Organic Nanoplatforms for Iodinated Contrast Media in CT Imaging. Molecules. 2021;26:7063
21. Yu H, Guo H, Wang Y, Wang Y, Zhang L. Bismuth nanomaterials as contrast agents for radiography and computed tomography imaging and their quality/safety considerations. WIREs Nanomedicine and Nanobiotechnology. 2022 14
22. Luo D, Wang X, Burda C, Basilion JP. Recent Development of Gold Nanoparticles as Contrast Agents for Cancer Diagnosis. Cancers (Basel). 2021;13:1825
23. Li Y, Zhang R, Xu Z, Wang Z. Advances in Nanoliposomes for the Diagnosis and Treatment of Liver Cancer. Int J Nanomedicine. 2022 Volume 17: 909-25
24. Yoo D, Jung W, Son Y, Jon S. Glutathione-Responsive Gold Nanoparticles as Computed Tomography Contrast Agents for Hepatic Diseases. ACS Appl Bio Mater. 2021;4:4486-94
25. Nievelstein RAJ, Quarles van Ufford HME, Kwee TC. et al. Radiation exposure and mortality risk from CT and PET imaging of patients with malignant lymphoma. Eur Radiol. 2012;22:1946-54
26. Wang Y, Yu B, Wang L. et al. 3D conditional generative adversarial networks for high-quality PET image estimation at low dose. Neuroimage. 2018;174:550-62
27. Lee M-Y, Choi D, Jang M-S, Lee JH. Biocompatible and Biodegradable Fe 3+ -Melanoidin Chelate as a Potentially Safe Contrast Agent for Liver MRI. Bioconjug Chem. 2018;29:2426-35
28. Thangudu S, Huang E-Y, Su C-H. Safe magnetic resonance imaging on biocompatible nanoformulations. Biomater Sci. 2022;10:5032-53
29. Neeley C, Moritz M, Brown JJ, Zhou Y. Acute side effects of three commonly used gadolinium contrast agents in the paediatric population. Br J Radiol [Internet]. 2016;89:20160027. Available at: http://www.birpublications.org/doi/10.1259/bjr.20160027
30. Zhang Y, Li X, Chen X. et al. Construction of ultrasmall gold nanoparticles based contrast agent via Host-Guest interaction for Tumor-targeted magnetic resonance imaging. Mater Des. 2022;217:110620
31. Aldenhoven L, Frotscher C, Körver-Steeman R. et al. Sentinel lymph node mapping with superparamagnetic iron oxide for melanoma: a pilot study in healthy participants to establish an optimal MRI workflow protocol. BMC Cancer. 2022;22:1062
32. Brito B, Price TW, Gallo J, Bañobre-López M, Stasiuk GJ. Smart magnetic resonance imaging-based theranostics for cancer. Theranostics. 2021;11:8706-37
33. Ash C, Dubec M, Donne K, Bashford T. Effect of wavelength and beam width on penetration in light-tissue interaction using computational methods. Lasers Med Sci. 2017;32:1909-18
34. Oberli MA, Schoellhammer CM, Langer R, Blankschtein D. Ultrasound-enhanced transdermal delivery: recent advances and future challenges. Ther Deliv. 2014;5:843-57
35. Tarighatnia A, Fouladi MR, Nader ND, Aghanejad A, Ghadiri H. Recent trends of contrast agents in ultrasound imaging: a review of the classifications and applications. Mater Adv. 2022;3:3726-41
36. Abedi MH, Yao MS, Mittelstein DR. et al. Ultrasound-controllable engineered bacteria for cancer immunotherapy. Nat Commun. 2022;13:1585
37. Dahan M, Cortet M, Lafon C, Padilla F. Combination of Focused Ultrasound, Immunotherapy, and Chemotherapy. Journal of Ultrasound in Medicine. 2023;42:559-73
38. Yan P, Liu L-H, Wang P. Sonodynamic Therapy (SDT) for Cancer Treatment: Advanced Sensitizers by Ultrasound Activation to Injury Tumor. ACS Appl Bio Mater. 2020;3:3456-75
39. Cotero V, Graf J, Miwa H. et al. Stimulation of the hepatoportal nerve plexus with focused ultrasound restores glucose homoeostasis in diabetic mice, rats and swine. Nat Biomed Eng. 2022;6:683-705
40. Kim MAh, Lee C-M. NIR-Mediated drug release and tumor theranostics using melanin-loaded liposomes. Biomater Res. 2022;26:22
41. Entzian K, Aigner A. Drug Delivery by Ultrasound-Responsive Nanocarriers for Cancer Treatment. Pharmaceutics. 2021;13:1135
42. Fu L, Ke H-T, Fu L, Ke H-T. Nanomaterials incorporated ultrasound contrast agents for cancer theranostics. Cancer Biol Med. 2016;13:313-24
43. Xu W, Dong C, Hu H. et al. Engineering Janus Chemoreactive Nanosonosensitizers for Bilaterally Augmented Sonodynamic and Chemodynamic Cancer Nanotherapy. Adv Funct Mater. 2021;31:2103134
44. Jiao X, Sun L, Zhang W. et al. Engineering oxygen-deficient ZrO2-x nanoplatform as therapy-activated “immunogenic cell death (ICD)” inducer to synergize photothermal-augmented sonodynamic tumor elimination in NIR-II biological window. Biomaterials. 2021;272:120787
45. Wang C, Chen X, Wang L. et al. Bioadhesive ultrasound for long-term continuous imaging of diverse organs. Science (1979). 2022;377:517-23
46. Ahn J, Kim JY, Choi W, Kim C. High-resolution functional photoacoustic monitoring of vascular dynamics in human fingers. Photoacoustics. 2021;23:100282
47. Huang J, Liu F, Han X. et al. Nanosonosensitizers for Highly Efficient Sonodynamic Cancer Theranostics. Theranostics. 2018;8:6178-94
48. Wood CA, Han S, Kim CS. et al. Clinically translatable quantitative molecular photoacoustic imaging with liposome-encapsulated ICG J-aggregates. Nat Commun. 2021;12:5410
49. Cheheltani R, Ezzibdeh RM, Chhour P. et al. Tunable, biodegradable gold nanoparticles as contrast agents for computed tomography and photoacoustic imaging. Biomaterials. 2016;102:87-97
50. Nguyen VP, Qian W, Li Y. et al. Chain-like gold nanoparticle clusters for multimodal photoacoustic microscopy and optical coherence tomography enhanced molecular imaging. Nat Commun. 2021;12:34
51. Farooq A, Sabah S, Dhou S, Alsawaftah N, Husseini G. Exogenous Contrast Agents in Photoacoustic Imaging: An In vivo Review for Tumor Imaging. Nanomaterials. 2022;12:393
52. Yan H, Chen J, Li Y. et al. Ultrasmall hybrid protein-copper sulfide nanoparticles for targeted photoacoustic imaging of orthotopic hepatocellular carcinoma with a high signal-to-noise ratio. Biomater Sci. 2019;7:92-103
53. Han Y, Yi H, Wang Y, Li Z, Chu X, Jiang J-H. Ultrathin Zinc Selenide Nanoplatelets Boosting Photoacoustic Imaging of In situ Copper Exchange in Alzheimer's Disease Mice. ACS Nano. 2022;16:19053-66
54. Alphandéry E. Iron oxide nanoparticles as multimodal imaging tools. RSC Adv. 2019;9:40577-87
55. Park E-Y, Oh D, Park S, Kim W, Kim C. New contrast agents for photoacoustic imaging and theranostics: Recent 5-year overview on phthalocyanine/naphthalocyanine-based nanoparticles. APL Bioeng. 2021;5:031510
56. Liu X, Duan Y, Liu B. Nanoparticles as contrast agents for photoacoustic brain imaging. Aggregate. 2021;2:4-19
57. Yamada H, Matsumoto N, Komaki T. et al. Photoacoustic in vivo 3D imaging of tumor using a highly tumor-targeting probe under high-threshold conditions. Sci Rep. 2020;10:19363
58. Kiru L, Zlitni A, Tousley AM. et al. In vivo imaging of nanoparticle-labeled CAR T cells. Proceedings of the National Academy of Sciences. 2022 119
59. Neprokin A, Broadway C, Myllylä T, Bykov A, Meglinski I. Photoacoustic Imaging in Biomedicine and Life Sciences. Life. 2022;12:588
60. Gao X, Chen X, Hu H. et al. A photoacoustic patch for three-dimensional imaging of hemoglobin and core temperature. Nat Commun. 2022;13:7757
61. Steelman ZA, Ho DS, Chu KK, Wax A. Light-scattering methods for tissue diagnosis. Optica. 2019;6:479
62. Guo Z, Cui Z. Fluorescent nanotechnology for in vivo imaging. WIREs Nanomedicine and Nanobiotechnology. 2021 13
63. Gil HM, Price TW, Chelani K, Bouillard J-SG, Calaminus SDJ, Stasiuk GJ. NIR-quantum dots in biomedical imaging and their future. iScience. 2021;24:102189
64. Gao Y, Liu Y, Yan R. et al. Bifunctional Peptide-Conjugated Gold Nanoparticles for Precise and Efficient Nucleus-Targeting Bioimaging in Live Cells. Anal Chem. 2020;92:13595-603
65. Ou X, Liu Y, Zhang M, Hua L, Zhan S. Plasmonic gold nanostructures for biosensing and bioimaging. Microchimica Acta. 2021;188:304
66. Si P, Razmi N, Nur O. et al. Gold nanomaterials for optical biosensing and bioimaging. Nanoscale Adv. 2021;3:2679-98
67. Wang S, Li B, Zhang F. Molecular Fluorophores for Deep-Tissue Bioimaging. ACS Cent Sci. 2020;6:1302-16
68. Singh S, Dhawan A, Karhana S, Bhat M, Dinda AK. Quantum Dots: An Emerging Tool for Point-of-Care Testing. Micromachines (Basel). 2020;11:1058
69. Bayal M, Chandran N, Pilankatta R, Nair SS. Semiconductor Quantum Dots and Core Shell Systems for High Contrast Cellular/Bio Imaging. In. 2021:27-38
70. Wu Y, Ali MRK, Chen K, Fang N, El-Sayed MA. Gold nanoparticles in biological optical imaging. Nano Today. 2019;24:120-40
71. Yajan P, Yulianto N, Saba M. et al. Intracellular gold nanoparticles influence light scattering and facilitate amplified spontaneous emission generation. J Colloid Interface Sci. 2022;622:914-23
72. Kim D, Aktalay A, Jensen N. et al. Supramolecular Complex of Photochromic Diarylethene and Cucurbit[7]uril: Fluorescent Photoswitching System for Biolabeling and Imaging. J Am Chem Soc. 2022;144:14235-47
73. Azhdarinia A, Daquinag AC, Tseng C. et al. A peptide probe for targeted brown adipose tissue imaging. Nat Commun. 2013;4:2472
74. Paulus A, Drude N, Nascimento EBM. et al. [18F]BODIPY-triglyceride-containing chylomicron-like particles as an imaging agent for brown adipose tissue in vivo. Sci Rep. 2019;9:2706
75. Munteanu R, Onaciu A, Moldovan C. et al. Adipocyte-Based Cell Therapy in Oncology: The Role of Cancer-Associated Adipocytes and Their Reinterpretation as Delivery Platforms. Pharmaceutics. 2020;12:402
76. Cao Y. Adipocyte and lipid metabolism in cancer drug resistance. Journal of Clinical Investigation. 2019;129:3006-17
77. Wen D, Wang J, van den Driessche G. et al. Adipocytes as Anticancer Drug Delivery Depot. Matter. 2019;1:1203-14
78. Mansouri M, Hussherr M-D, Strittmatter T. et al. Smart-watch-programmed green-light-operated percutaneous control of therapeutic transgenes. Nat Commun. 2021;12:3388
79. Lifante J, Shen Y, Ximendes E, Martín Rodríguez E, Ortgies DH. The role of tissue fluorescence in in vivo optical bioimaging. J Appl Phys. 2020;128:171101
80. Bon P, Cognet L. On Some Current Challenges in High-Resolution Optical Bioimaging. ACS Photonics. 2022;9:2538-46
81. Wang R, Zhou L, Wang W, Li X, Zhang F. In vivo gastrointestinal drug-release monitoring through second near-infrared window fluorescent bioimaging with orally delivered microcarriers. Nat Commun. 2017;8:14702
82. Xie Q, Liu J, Chen B. et al. NIR-II Fluorescent Activatable Drug Delivery Nanoplatform for Cancer-Targeted Combined Photodynamic and Chemotherapy. ACS Appl Bio Mater. 2022;5:711-22
83. Zeng C, Ouyang J, Sun L. et al. An activatable probe for detection and therapy of food-additive-related hepatic injury via NIR-II fluorescence/optoacoustic imaging and biomarker-triggered drug release. Anal Chim Acta. 2022;1208:339831
84. Zhong Y, Ma Z, Zhu S. et al. Boosting the down-shifting luminescence of rare-earth nanocrystals for biological imaging beyond 1500 nm. Nat Commun. 2017;8:737
85. Fan Y, Wang P, Lu Y. et al. Lifetime-engineered NIR-II nanoparticles unlock multiplexed in vivo imaging. Nat Nanotechnol. 2018;13:941-6
86. Domingo-Lopez DA, Lattanzi G, Schreiber LHJ. et al. Medical devices, smart drug delivery, wearables and technology for the treatment of Diabetes Mellitus. Adv Drug Deliv Rev. 2022;185:114280
87. Han JJ, Graham JH, Snyder DI, Alfieri T. Long-term Use of Wearable Health Technology by Chronic Pain Patients. Clin J Pain. 2022;38:701-10
88. Low CA. Harnessing consumer smartphone and wearable sensors for clinical cancer research. NPJ Digit Med. 2020;3:140
89. Beauchamp UL, Pappot H, Holländer-Mieritz C. The Use of Wearables in Clinical Trials During Cancer Treatment: Systematic Review. JMIR Mhealth Uhealth. 2020;8:e22006
90. Duncker D, Ding WY, Etheridge S. et al. Smart Wearables for Cardiac Monitoring—Real-World Use beyond Atrial Fibrillation. Sensors. 2021;21:2539
91. Bayoumy K, Gaber M, Elshafeey A. et al. Smart wearable devices in cardiovascular care: where we are and how to move forward. Nat Rev Cardiol. 2021;18:581-99
92. Long Y, Li J, Yang F, Wang J, Wang X. Wearable and Implantable Electroceuticals for Therapeutic Electrostimulations. Advanced Science. 2021;8:2004023
93. Araromi OA, Graule MA, Dorsey KL. et al. Ultra-sensitive and resilient compliant strain gauges for soft machines. Nature. 2020;587:219-24
94. Rykov Y, Thach T-Q, Bojic I, Christopoulos G, Car J. Digital Biomarkers for Depression Screening With Wearable Devices: Cross-sectional Study With Machine Learning Modeling. JMIR Mhealth Uhealth. 2021;9:e24872
95. Sharifi S, Hajipour MJ, Gould L, Mahmoudi M. Nanomedicine in Healing Chronic Wounds: Opportunities and Challenges. Mol Pharm. 2021;18:550-75
96. Oliva N, Almquist BD. Spatiotemporal delivery of bioactive molecules for wound healing using stimuli-responsive biomaterials. Adv Drug Deliv Rev. 2020;161-162:22-41
97. Zhu Q, Hong Y, Huang Y. et al. Polyglutamic Acid-Based Elastic and Tough Adhesive Patch Promotes Tissue Regeneration through In situ Macrophage Modulation. Advanced Science. 2022;9:2106115
98. Xiong Z, Achavananthadith S, Lian S. et al. A wireless and battery-free wound infection sensor based on DNA hydrogel. Sci Adv. 2021 7
99. Bhubhanil S, Talodthaisong C, Khongkow M. et al. Enhanced wound healing properties of guar gum/curcumin-stabilized silver nanoparticle hydrogels. Sci Rep. 2021;11:21836
100. Fang X, Wang C, Zhou S. et al. Hydrogels for Antitumor and Antibacterial Therapy. Gels. 2022;8:315
101. Ochoa M, Rahimi R, Zhou J. et al. Integrated sensing and delivery of oxygen for next-generation smart wound dressings. Microsyst Nanoeng. 2020;6:46
102. Guan Y, Niu H, Liu Z. et al. Sustained oxygenation accelerates diabetic wound healing by promoting epithelialization and angiogenesis and decreasing inflammation. Sci Adv. 2021 7
103. Akhmetova A, Heinz A. Electrospinning Proteins for Wound Healing Purposes: Opportunities and Challenges. Pharmaceutics. 2020;13:4
104. Zheng Y, Hong X, Wang J. et al. 2D Nanomaterials for Tissue Engineering and Regenerative Nanomedicines: Recent Advances and Future Challenges. Adv Healthc Mater. 2021;10:2001743
105. Gao L, Zhou Y, Peng J. et al. A novel dual-adhesive and bioactive hydrogel activated by bioglass for wound healing. NPG Asia Mater. 2019;11:66
106. Cutting KF. Wound exudate: composition and functions. Br J Community Nurs. 2003;8:S4-9
107. Zhu G, Wang Q, Lu S, Niu Y. Hydrogen Peroxide: A Potential Wound Therapeutic Target. Medical Principles and Practice. 2017;26:301-8
108. Hu M, Korschelt K, Daniel P, Landfester K, Tremel W, Bannwarth MB. Fibrous Nanozyme Dressings with Catalase-Like Activity for H 2 O 2 Reduction To Promote Wound Healing. ACS Appl Mater Interfaces. 2017;9:38024-31
109. Wu K, Wu X, Chen M, Wu H, Jiao Y, Zhou C. H2O2-responsive smart dressing for visible H2O2 monitoring and accelerating wound healing. Chemical Engineering Journal. 2020;387:124127
110. Luo R, Dai J, Zhang J, Li Z. Accelerated Skin Wound Healing by Electrical Stimulation. Adv Healthc Mater. 2021;10:2100557
111. Tan M, Xu Y, Gao Z. et al. Recent Advances in Intelligent Wearable Medical Devices Integrating Biosensing and Drug Delivery. Advanced Materials. 2022;34:2108491
112. Wang K, Parekh U, Ting JK. et al. A Platform to Study the Effects of Electrical Stimulation on Immune Cell Activation During Wound Healing. Adv Biosyst. 2019;3:1900106
113. Leal J, Jedrusik N, Shaner S, Boehler C, Asplund M. SIROF stabilized PEDOT/PSS allows biocompatible and reversible direct current stimulation capable of driving electrotaxis in cells. Biomaterials. 2021;275:120949
114. Liu S, Li D, Wang Y. et al. Flexible, high-strength and multifunctional polyvinyl alcohol/MXene/polyaniline hydrogel enhancing skin wound healing. Biomater Sci. 2022;10:3585-96
115. Jeong S-H, Lee Y, Lee M-G, Song WJ, Park J-U, Sun J-Y. Accelerated wound healing with an ionic patch assisted by a triboelectric nanogenerator. Nano Energy. 2021;79:105463
116. Sharma A, Panwar V, Mondal B. et al. Electrical stimulation induced by a piezo-driven triboelectric nanogenerator and electroactive hydrogel composite, accelerate wound repair. Nano Energy. 2022;99:107419
117. Dong R, Guo B. Smart wound dressings for wound healing. Nano Today. 2021;41:101290
118. Xu G, Lu Y, Cheng C. et al. Battery-Free and Wireless Smart Wound Dressing for Wound Infection Monitoring and Electrically Controlled On-Demand Drug Delivery. Adv Funct Mater. 2021;31:2100852
119. Mao L, Hu S, Gao Y. et al. Biodegradable and Electroactive Regenerated Bacterial Cellulose/MXene (Ti 3 C 2 T x ) Composite Hydrogel as Wound Dressing for Accelerating Skin Wound Healing under Electrical Stimulation. Adv Healthc Mater. 2020;9:2000872
120. Hada V, Malvi D, Mili M. et al. MXenes: promising 2D materials for wound dressing applications - a perspective review. Mater Adv. 2022;3:7445-62
121. Kalidasan V, Yang X, Xiong Z. et al. Wirelessly operated bioelectronic sutures for the monitoring of deep surgical wounds. Nat Biomed Eng. 2021;5:1217-27
122. Breit S, Kupferberg A, Rogler G, Hasler G. Vagus Nerve as Modulator of the Brain-Gut Axis in Psychiatric and Inflammatory Disorders. Front Psychiatry. 2018 9
123. González HFJ, Yengo-Kahn A, Englot DJ. Vagus Nerve Stimulation for the Treatment of Epilepsy. Neurosurg Clin N Am. 2019;30:219-30
124. Kamel LY, Xiong W, Gott BM, Kumar A, Conway CR. Vagus nerve stimulation: An update on a novel treatment for treatment-resistant depression. J Neurol Sci. 2022;434:120171
125. Wang Y, Zhan G, Cai Z. et al. Vagus nerve stimulation in brain diseases: Therapeutic applications and biological mechanisms. Neurosci Biobehav Rev. 2021;127:37-53
126. Pavlov VA, Tracey KJ. The vagus nerve and the inflammatory reflex—linking immunity and metabolism. Nat Rev Endocrinol. 2012;8:743-54
127. Caravaca AS, Gallina AL, Tarnawski L. et al. Vagus nerve stimulation promotes resolution of inflammation by a mechanism that involves Alox15 and requires the α7nAChR subunit. Proceedings of the National Academy of Sciences. 2022 119
128. van Leusden JWR, Sellaro R, Colzato LS. Transcutaneous Vagal Nerve Stimulation (tVNS): a new neuromodulation tool in healthy humans? Front Psychol. 2015 6
129. Bolz A, Bolz L-O. Technical aspects and future approaches in transcutaneous vagus nerve stimulation (tVNS). Autonomic Neuroscience. 2022;239:102956
130. Goggins E, Mitani S, Tanaka S. Clinical perspectives on vagus nerve stimulation: present and future. Clin Sci. 2022;136:695-709
131. Chen P, Wang Q, Wan X. et al. Wireless electrical stimulation of the vagus nerves by ultrasound-responsive programmable hydrogel nanogenerators for anti-inflammatory therapy in sepsis. Nano Energy. 2021;89:106327
132. de Lartigue G. Role of the vagus nerve in the development and treatment of diet-induced obesity. J Physiol. 2016;594:5791-815
133. Pavlov VA. The evolving obesity challenge: targeting the vagus nerve and the inflammatory reflex in the response. Pharmacol Ther. 2021;222:107794
134. Arner P, Bernard S, Salehpour M. et al. Dynamics of human adipose lipid turnover in health and metabolic disease. Nature. 2011;478:110-3
135. Uppot RN. Technical challenges of imaging & image-guided interventions in obese patients. Br J Radiol. 2018 20170931
136. Li J, Cha R, Luo H, Hao W, Zhang Y, Jiang X. Nanomaterials for the theranostics of obesity. Biomaterials. 2019;223:119474
137. Zhang X, Zhang Z, Diao W. et al. Early-diagnosis of major depressive disorder: From biomarkers to point-of-care testing. TrAC Trends in Analytical Chemistry. 2023;159:116904
138. Parlak O, Keene ST, Marais A, Curto VF, Salleo A. Molecularly selective nanoporous membrane-based wearable organic electrochemical device for noninvasive cortisol sensing. Sci Adv. 2018 4
139. Parlak O. Portable and wearable real-time stress monitoring: A critical review. Sensors and Actuators Reports. 2021;3:100036
140. Tang W, Yin L, Sempionatto JR, Moon J, Teymourian H, Wang J. Touch-Based Stressless Cortisol Sensing. Advanced Materials. 2021;33:2008465
141. Mishra S. Electroceuticals in medicine - The brave new future. Indian Heart J. 2017;69:685-6
142. Yap JYY, Keatch C, Lambert E, Woods W, Stoddart PR, Kameneva T. Critical Review of Transcutaneous Vagus Nerve Stimulation: Challenges for Translation to Clinical Practice. Front Neurosci. 2020 14
143. Rahman MdA, Tharu NS, Gustin SM, Zheng Y-P, Alam M. Trans-Spinal Electrical Stimulation Therapy for Functional Rehabilitation after Spinal Cord Injury: Review. J Clin Med. 2022;11:1550
144. Tong W, Meffin H, Garrett DJ, Ibbotson MR. Stimulation Strategies for Improving the Resolution of Retinal Prostheses. Front Neurosci. 2020 14
145. ZHAO Z, SUN W, ZHAO X. et al. Stimulation of both inspiratory and expiratory muscles versus diaphragm-only paradigm for rehabilitation in severe chronic obstructive pulmonary disease patients: a randomized controlled pilot study. Eur J Phys Rehabil Med. 2022 58
146. da Silva ML, Chiappa GR, da Silva VM. et al. Effect of transcutaneous electrical nerve stimulation on peripheral to central blood pressure ratio in healthy subjects. Clin Physiol Funct Imaging. 2016;36:293-7
147. Ramadi KB, Srinivasan SS, Traverso G. Electroceuticals in the Gastrointestinal Tract. Trends Pharmacol Sci. 2020;41:960-76
148. Chen P, Wang Q, Wan X. et al. Wireless electrical stimulation of the vagus nerves by ultrasound-responsive programmable hydrogel nanogenerators for anti-inflammatory therapy in sepsis. Nano Energy. 2021;89:106327
149. Long Y, Li J, Yang F, Wang J, Wang X. Wearable and Implantable Electroceuticals for Therapeutic Electrostimulations. Advanced Science. 2021;8:2004023
150. Kireev D, Sel K, Ibrahim B. et al. Continuous cuffless monitoring of arterial blood pressure via graphene bioimpedance tattoos. Nat Nanotechnol. 2022;17:864-70
151. Singh V, Kesharwani P. Recent advances in microneedles-based drug delivery device in the diagnosis and treatment of cancer. Journal of Controlled Release. 2021;338:394-409
152. Wang Z, Yang Z, Jiang J. et al. Silk Microneedle Patch Capable of On-Demand Multidrug Delivery to the Brain for Glioblastoma Treatment. Advanced Materials. 2022;34:2106606
153. Abramson A, Chan CT, Khan Y. et al. A flexible electronic strain sensor for the real-time monitoring of tumor regression. Sci Adv. 2022 8
154. Rastogi A, Yadav K, Mishra A. et al. Early diagnosis of lung cancer using magnetic nanoparticles-integrated systems. Nanotechnol Rev. 2022;11:544-74
155. Kireev D, Sel K, Ibrahim B. et al. Continuous cuffless monitoring of arterial blood pressure via graphene bioimpedance tattoos. Nat Nanotechnol. 2022;17:864-70
156. Yeh BM, FitzGerald PF, Edic PM. et al. Opportunities for new CT contrast agents to maximize the diagnostic potential of emerging spectral CT technologies. Adv Drug Deliv Rev. 2017;113:201-22
Author contact
![]() Corresponding author: Amit Kumar Sharma. Email: sharmaamitkumarnthu.edu.tw
Corresponding author: Amit Kumar Sharma. Email: sharmaamitkumarnthu.edu.tw

 Global reach, higher impact
Global reach, higher impact