ISSN: 2206-7418
Nanotheranostics 2023; 7(4):450-459. doi:10.7150/ntno.86618 This issue Cite
Editorial
Bench to Beside Technology: Nanobios Lab Industry Academia Translational Bridge
1. Department of Biosciences and Bioengineering, Indian Institute of Technology, Bombay, Powai, 76001, India.
2. School of Biochemical Engineering, Indian Institute of Technology (BHU), Varanasi 221005, India.
Received 2023-5-29; Accepted 2023-5-31; Published 2023-9-1
Abstract
In the biomedical field, translational research has become a prior need for bridging the gap between industry and academia for the budding entrepreneurs and scientists. Translational research has various interdisciplinary scopes, but it is complex, unlike other non-medical product developments, as it deals with lives (both animals and humans). For developing bench to beside technologies, various factors such as repeatability, reliability, scalability, compatibility, compliance, adverse effects, regulatory approvals, relevance commercial potential and many more human factors need to be explored. Apart from these, other aspects like drug design/or development, bioengineering approaches, chemical synthesis methodologies, choice of diagnostics and therapeutics kit or tool, supply industries, nano(bio)technology, tissue printing, diagnostics and many others need to be studied. To the best of our knowledge, an academia-industry partnership and multidisciplinary collaborations may reduce various related hurdles. Among 200 research groups/laboratories, NanoBios has emerged as a pathbreaker in this field based on its diverse portfolio, world class resources, co-existence of various collaborative facilities, pool of scholars, industry partners, funding, and all other decorations required for exemplary translational research works. Commercial exploitation incentivizes innovations, and the lab has created an impact in the Indian startup space within a decade by transferring many technologies to the industries, ultimately incubating an entrepreneurial environment within an academic institute where various stakeholders benefit from each other as well as meet societal needs by generating indigenous translational products.
Keywords: Bench to Beside Technology, Nanobios Lab, Industry-Academia Collaboration, Translational Research, Biomedical Innovations, Multidisciplinary Collaborations, Entrepreneurship in Academia, Interdisciplinary Scope, Regulatory Approvals, Relevance and Commercial Potential, Bioengineering Approaches, Drug Design and Development, Chemical Synthesis Methodologies, Diagnostics and Therapeutics, Nano(bio)technology, Tissue Printing, Supply Industries, Indian Institute of Technology, Academia-Industry Partnership, Exemplary Translational Research, Startup Ecosystem, Indigenous Translational Products, Societal Impact, Funding and Resources, Pool of Scholars, Incubating Entrepreneurship.
Importance of Industry-Academia Bridge
Lifelong learning is the 'Mantra' for survival in this highly competitive world of information and automation. Today's researchers are multitaskers and prefer to outsource professional services for better and rapid outcomes. Increased competition and globalization in the engineering community motivate business and academic organizations to increase their collaboration. Doing practically useful and scientifically rigorous research while having a substantial societal impact is difficult for academics and institutions [1,2,3]. It may be challenging to manage collaboration among organizations with diverse viewpoints; it requires knowledge and competence to fulfil differing expectations. Various practice-oriented methods, such as collaboration research [4], action research [5], interactive research [6], and engaged research, have been practiced for the execution of standard bio-design models for medical device development.
Infrastructure
Infrastructure establishment is always expensive, and lack of it can hinder the growth of any research, especially in the translational field. In institutions like the Indian Institute of Technology, Bombay, researchers are allowed to move from universities to corporations for a certain duration on sabbaticals where such migration helps the researcher to absorb new skills and knowledge, and the industry gets benefits out of the fresh ideas and innovative solutions from the academic person onboard.
Scaling up
In fundamental research, new knowledge emerges through rigorous methodologies over a long time with great uncertainties. Knowledge and understanding do not follow a linear relationship. In translational research, the venture starts with a problem statement about a particular industrial application. Therefore, from the beginning, the research gets a flow diagram involving proof-of-concept generation, prototyping, validation, and scaling up. From a manufacturing point of view, prototype-to-product conversion is much more difficult. It has a 'deep valley of death' that means a high chance of failure. A manufacturing or industry partner who warns the team about on-ground challenges of mass production in every step of productization becomes a valuable member of the co-creation team. This can escalate the technology readiness level (TRL) and gives a destination to innovative ideas. There are various models of such collaborations. In the NanoBios lab, various collaboration models have been adopted for translational works. A few examples are mentioned below:
Resource sharing
(a) Co-develop / Co-creation model where both Nanobios lab and industry jointly invest various resources to develop a product.
(b) Outsourcing by the industry to NanoBios lab
(c) Technology transfer/ Licensing model: both early (TRL-3-5) and late TRL prototypes were licensed to domain industries.
(d) Corporate social responsibility model: for a societal cause industry donate funds for a noble and innovative societal innovation.
(e) Grant/ funding model for new technology exploration.
Outsourcing
(f) Consultancy for an existing product or service assigned to the lab by industry
(g) Technology assessment of a new product
Knowledge partnership
(h) Internship model: both-way student/ employee exchange occurs for knowledge and practical skills development.
(i) Scholarship model: industry-sponsored researchers work in the lab to work for a given project.
(j) Logistics partnerships: chemicals, ingredients, components and other logistics support occur through mutual trust-building of the pharmaceutical and chemical vendors (industry), and this is a very vital/ key partnership.
Validation partnership
(k) Human and Animal trial partnerships: various clinical trial agencies collaborate with the lab vis-à-vis.
(l) Validation and Standardisation services related partnerships: after the technology is developed, it has to be validated before going into clinical trials or maybe to market. Therefore, various testing and validation industries partner with the lab for such purposes. Both preliminary and professional validation are important as they reduce failure rate post-marketing and determine the correct way translation should follow.
Technical partnership
Various industries offer rapid prototyping tools, batch manufacturing, market survey, statistics, internet of things enabling, app development and many other technical aspects, which are integral to the translational research process.
In a nutshell, multi/cross-platform research works like the works of Prof Rohit Srivastava. Various industries play an important role in designing and developing a product, which further goes into validation through clinical trials. Therefore, many collaborations are required through personal and official contacts to facilitate the need to utilize certain instruments, facilities, devices, and services or acquire hands-on skills. The amalgamation of the industry with academia accelerates the developmental process and shortens the product development cycle.
Healthcare establishments
At the core of translational research in the biomedical field are the establishments that play the biggest role in germinating, identifying, and projecting need statements, screening, and validation. Finally, problem statement identifications include hospitals/ clinics and healthcare workers. The ideas from real-life practitioners are validated through their vast experiences. When an engineer makes a product in healthcare without directly or indirectly involving a clinician in the team, he ends up making some solution to a hypothetical problem. Through IIT Bombay, many healthcare industries like nearby hospitals, for instance SION hospital, Hiranandani hospital, DYPUSM (medical college and hospital), Tata memorial hospital (TMH), KEM hospital, and many elite healthcare establishments are partners of such translational research from the early days of NanoBios lab. Though the primary focus is on Nanotechnology, the lab has brought in industry-sponsored projects in maternal and child health, cancer theranostic, point-of-care diagnostics, biodegradable plastic, remote healthcare monitoring devices, protective equipment for Covid management, telemedicine, and in various other diverse domains. The benefits of bringing in the industry in various stages of translational research are:
(a) Expansion of networks for both industry and academic institutes
(b) flexibility in working in both places
(c) need quantification and commercial potential become apparent.
(d) establishment and infrastructural cost saving.
(e) early licensing agreements with the industry give a destination to the idea.
(f) industry saves money, time, and human resources by outsourcing the research to a reliable knowledge partner (academia)
(g) dogmatic scientific perspectives are overcome.
(h) possibilities of exploring the eco-space of commercialization of a research product.
(i) hands-on practices.
(j) the practice of landmark/ deliverable-wise division of targets.
(k) industrial acculturation.
(l) TRL conversion.
(m) Ability to work in a team.
(n) time and resource management practices.
(o) coordination skill development.
(p) exposure to current industry trends.
Translation of Ideas into Products
Research in the field of life sciences is booming, and many devices and therapeutic approaches are being developed as a direct result. Nonetheless, numerous problems in the healthcare system have not yet been addressed. Sometimes, even if a remedy does exist, it may be too costly or unavailable on a global scale. as a result, there is a pressing requirement for the creation of reasonably priced and widely available medical/ healthcare devices. Nano bios lab, headed by Prof Rohit Srivastava, is well-known for developing a wide range of biomedical products, including screening devices and therapeutic agents with a focus on resource-limited healthcare settings in various domains like malnutrition, maternal and child health, diabetes, dyslipidemia, tuberculosis, cancer and many others. These technologies are locally sourced, inexpensive, portable, and simple to implement on a large scale.
Microneedle Patch for pain management
To name a few, Prof Srivastava's lab has developed a novel method to develop a polymeric microneedle patch for transdermal drug delivery. We have used polyvinyl alcohol (PVA) material. This was done by soldering microsize headpins onto a Veroboard using a soldering station to prepare the master mold. The microneedle patch was tested for drug delivery using Rhodamine 6G (model drug). The tips of the microneedles were coated with Dye and pierced porcine skin. Most of the drug was delivered within 12 Hours. This technology has been transferred to a company for many applications, including wound care.
Point of care lipid profile test
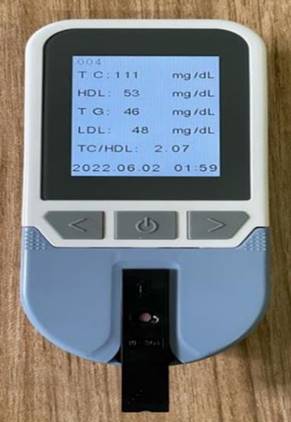
CholchekTM (supported by BIRAC) was another excellent innovation to determine the body's total cholesterol, HDL, LDL and triglyceride levels. It uses an enzymatic paper-based colorimetric assay to determine all the by-products. This technology has immense potential as a standalone device in Tier 2 and Tier 3 cities of India, whereby results can be available to the patient within half an hour.
CholchekTM is a device for measuring systemic Total Cholesterol, HDL, LDL & Triglyceride levels in the body (image from actual device taken through author's mobile phone)
Point-of-care test for detecting Myocardial Infarction
Tropchek was another novel, non-invasive point-of-care in-vitro diagnostic device for detecting cardiac Troponins T and I in the blood (supported by ICMR). The academic clinical validation of the product demonstrated an assay LOD equivalent to commercial products and has tremendous potential for being launched as an indigenous product by our lab.
Point-of-care test for Diabetes Mellitus
NanoBios Lab has developed another in-vitro diagnostic tool for the detection of glycated forms of serum albumin. It is estimated that gestational diabetes may lead to severe detrimental consequences for both the mother and the fetus. Glycated albumin is a 3-week biomarker that is unaffected by pregnancy. This point-of-care test for glycated albumin is unavailable in India and is being developed in NanoBios Lab. This will particularly be useful in cases where HbA1C reading cannot be used due to already low Hb levels in anemic patients. This technology is also being transferred to a startup out of Nanobios Lab.
Bone and soft-tissue fixation device for orthopedic applications
Startups being highly encouraged at NanoBios, an exceptional low-cost indigenously developed bioresorbable bone screw for bone and soft-tissue fixation applications was fabricated by students along with Prof Bellare and Prof. Srivastava and a startup, Effecmed Pvt Ltd setup in SINE, IIT Bombay. Owning its unique properties, it was successfully patented (Application No. 201611012973). Collaborators propose to file another patent for an improved design of bone screws for better performance in the human body.
Covid-19 related works
The global COVID-19 pandemic shook the world and stalled many scientific expeditions leading to a rising burden on the healthcare sector. Still, NanoBios contributed to preventing this lethal viral infection and paved a new path toward developing bio-protective face masks. Navrakshak FFP2 and FFP-3 medical mask was developed with remarkable antimicrobial properties and later transferred to Paayas Healthcare Ltd. Along with this, affordable remote health monitoring devices like a low-cost, user-centric digital stethoscope with application software have been developed in collaboration with Indian Navy, and the technology has been licensed to Yashraj Biotech Ltd. A transitional and extremely affordable Intensive care unit monitoring system has been developed under Corporate social responsibility funding from Getinge India Pvt Ltd. IV fluid alarm system, continuous monitoring internet of things based pulse oximeter, digital therapeutic mobile applications, AI/ ML based fluid flow rate measurement systems have been developed for pandemic readiness.
Device for the prevention of implant-related infections
NanoBios also dived into solving medical concerns with high efficiency and product utility rates. The contribution towards developing Chitosan-based bioengineered localized delivery platforms for the prevention and treatment of orthopedic implant-associated infections cannot be ignored. This development was further transferred to Yashraj Biotech Ltd. for scaling up, manufacturing, clinical trials and subsequent marketing.
Miscellaneous
Other technologies and products by NanoBios are as follows:
- An on-demand, smart, and wearable drug delivery device for pain management with a controlled and sustained release for the treatment of arthritis.
- A bioengineered localized delivery system for managing post-surgical complications in oral and maxillofacial surgeries using a developed multifunctional (antibacterial agent, NSAIDs, analgesic and hemostatic agent) gel foam.
- A lateral flow assay (LFA) based point-of-care (POC) device for the detection of ferritin levels in the blood for screening of macrophage activation syndrome (MAS) arising due to rheumatic disorders.
- A portable device for micronutrient deficiency screening based on urinary iodine levels.
- Nanoparticles-based photothermal therapy as a potent cancer treatment developed in collaboration with Dr Abhijit De at ACTREC, Navi Mumbai.
- BF2 smaragdine-loaded liposomes as theranostic agents for bioimaging and photothermal therapy-based breast cancer treatment.
Bio-entrepreneurship Initiative
In the current state of development at the national and international levels, there is a steady rise in entrepreneurial activities across all sectors of the economy. Most of these ventures provide solutions in high-demand domains, including finance, artificial intelligence, big data, and healthcare technologies. No matter the state of the economy, healthcare will always be an important sector, as highlighted by the COVID-19 pandemic. This is why healthcare entrepreneurship or 'Bio-entrepreneurship' is on the rise across the globe. For bio-related entrepreneurial initiatives to succeed, there is a need to establish close collaborations between the stakeholders involved - hospitals, industry, governing bodies, patients, academia, and other research institutions.
NanoBios is one of the pioneers in India to lead this initiative of actively bridging the gap between industry and academia and facilitating close partnerships with hospitals for the effective development of therapeutics and other medical solutions. NanoBios benefits from the connections, resources, and expertise of its partners and collaborating institutions like Cipla, Zimmer and Peacock, Getinge India Ltd., Kryo CS Pvt. Ltd., Scion Agricos, YBL Ltd., Swaps Couture Ltd., Paayas Inc. Ltd., INMAS (DRDO), CBMR, and the Indian Navy. Many technologies developed at NanoBios have been transferred to and commercialized by existing industries and new startups by students (Biosense, Technologies Pvt. Ltd., CareNx Pvt. Ltd., Dynasense Technologies Pvt. Ltd., Qawach Bio Pvt Ltd, Nedocs Pvt Ltd, Nordetect Pvt Ltd, Effecmed Pvt Ltd and Arthritis Research Pvt. Ltd and many others).
Challenges in translational research
The research over the past two decades has shown that nanomaterials offer exciting opportunities in theranostics [7]. Despite the promising results in the preclinical studies, the clinical translation of nanomaterials from bench to bedside faces several challenges, such as difficulty in scale-up, fluctuating concentrations of payload at the target site, and rapid clearance by the reticuloendothelial system [8]. While addressing the challenges in the clinical translation of nanomaterials, it is to be noted that reducing adverse effects alone might not be sufficient for successful translation.
The global market for nanomaterials for biomedical applications is vast and still increasing [9]. However, there exists an imbalance between the number of preclinical studies and their translation into marketed pharmaceutical products [10]. This is mainly because of the considerable obstacles faced by nanomaterials in (i) the production techniques which satisfy the standards of 'Good manufacturing practices (GMP)' pertaining to reproducibility, techniques and cost, quality control assays for characterization such as size, polydispersity, morphology, charge, encapsulation, surface modifications, purity and stability; (ii) the streamlining of preclinical assays specifically for nanomaterials by designing and developing specialized toxicology assays, understanding the interactions of nanomaterials with the cellular and subcellular structures, their toxicity implications, maintaining structural stability following in vivo administration with limited accumulation in non-target organs; (iii) the streamlining of clinical evaluation for commercialization by establishing clear regulatory guidelines specifically for nanomaterials use with evidence based support from the studies on biological interaction of nanomaterials with the different organs and organ systems of patients [11]. Compared to conventional drug formulations such as tablets, capsules, injections, etc., nanomedicines' production is complex, expensive, and time-consuming. In addition to the general pharmaceutical standards, nanomaterials require new analytical tools and standardized methods to evaluate the key physical characteristics of nanomaterials.
Since nanomaterials exhibit unique physicochemical properties, their evaluation by regulatory bodies is quite complex [12] Moreover, the manufacturing process can be multifaceted, and there is considerable confusion over nanomedicines' interchangeability and substitutability those similar pharmacokinetic properties [13]. Unfortunately, the large gap between the scientific and regulatory bodies hampers the commercialization of nanomaterials. The establishment of clear regulatory and safety guidelines can make the process faster. Currently, nanomaterials are evaluated by FDA, TGA, and EMA [14]. However, the evaluation of the safety and efficacy requires well-established dedicated global regulatory standards and framework which assess the doses, administration routes, dosing frequency, and proposed clinical use with attention to complexity, route of administration, pharmacokinetics, pharmacodynamics and safety profile, and information on the most appropriate clinical trial design and patient selection [15]. However, while devising the guidelines, there should not be over-regulation which may hinder innovation progress.
Although FDA has approved several nanomaterials for theragnostic applications, there lacks a standard for evaluating the efficacy and safety or guidelines for using their nano-similar forms. Furthermore, medical practitioners must be given guidelines for selecting, handling, and substituting nanomedicines [16]. The existing guidelines by the International Pharmaceutical Federation (FIP guidelines) should be extended to include nanomedicines and nano-similar forms.
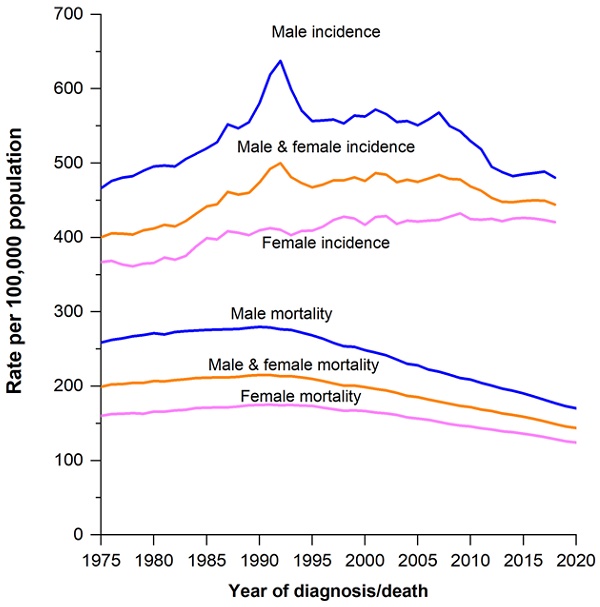
Importance of Early-Stage Diagnosis
Many common life-altering diseases and medical conditions can be effectively prevented, treated or controlled if diagnosed early, thus improving survival rates. According to the Surveillance, Epidemiology, and End Results (SEER) Program of the NIH, the 5-year relative survival rate of cancer cases recorded between 2012 to 2018 in the U.S. was 68.1% [17]. Figure 2 shows the modeled trend of new cancer case rates and the deaths in the U.S. between 1975 and 2019 for all cancer types and both sexes. Early diagnosis of bowel, breast and ovarian cancers increases survival by at least five years [18]. For diseases like type 2 diabetes increases the risk of developing other health conditions, including cardiovascular, kidney, and liver diseases [19]. Early diagnosis of diabetes would enable patients to take preventive measures before it is too late. For conditions like dementia commonly caused by Alzheimer's, early diagnosis allows the adoption of preventive measures to slow the progression of the disease. This allows patients and their families to make informed decisions, improving their quality of life [20]. For early screening and diagnosis to become the norm, such options must be more accessible and affordable, and adequate awareness must be spread to the masses. One of NanoBios' main foci is developing economic screening devices. For diabetes monitoring, for example, in collaboration with Biosense Technologies Private Limited, NanoBios Lab developed uChek, a urine diagnosis system based on the microalbumin to creatine ratio, and SYNC Glucometer, a compact glucometer for personal use with Bluetooth connectivity which allows you to visualize the user's blood glucose levels along with meal and exercise trends via a mobile application. Similarly, for non-invasive anemia detection, Biosense and NanoBios developed ToucHb, which screens detecting pallor in the conjunctiva, tongue and nail bed using a mobile application.
Other devices developed by NanoBios include UridsaTM, a colorimetry-based device for detecting preeclampsia and chronic kidney disease, and ElectrofinderTM, an optical reader and assay for detecting Na+ and K+ blood electrolytes. Apart from this, NanoBios also has different devices and technologies currently under clinical validation. These include the following:
• CholChekTM is an optical reader and assays for HDL, LDL, triglycerides, and total cholesterol.
• An insulin infusion pump using microneedle patches for continuous metered insulin infusion.
• A smartphone-based reader and image processing application to measure blood glycated hemoglobin (HbA1c).
• PorFloRTM, a fluorescence reader and strips for the detection of orthopedic implant-associated infection biomarkers such as C-reactive protein (CRP) and interleukin-6 (IL-6).
• Affordable, rapid and portable point-of-care lateral flow immunoassays for the detection of Vitamin B12 and Vitamin D3.
• A low-cost, commercially viable, lateral flow immunoassay-based point-of-care device for detecting TSH, T3, T4, Troponin I & T.
• A liquid reagent-based assay for urinary iodine, urinary Myo-inositol and creatinine for gestational diabetes.
• A low-cost and affordable T.B. diagnostics lateral flow assays in sputum, urine and blood.
Trends in cancer incidence (1975-2019) and mortality (1975-2020) rates by sex Reused with permission from CA A Cancer J Clinicians, 2023, 73,1, 17-48, DOI: 10.3322/caac.21763. (Graph created by plotting data on MS Excel)
Nano-contrast agent which completed clinical trials
| Agent | Clinical trial id | Functional modification | Remarks | Reference |
|---|---|---|---|---|
| Silica | NCT01266096 | 124I and Cy5, PET and optical | Define sentinel lymph nodes | [38] |
| Iron oxide | NCT01169935 | Ferumoxide, MRI | Identify the presence of implanted stem cells | [39] |
| Silica Nanoparticles | NCT02106598 | fluorescent cRGDY-PEG-Cy5.5-C dots | Real-Time Image-Guided Intraoperative Mapping of Nodal Metastases | [40] |
| Carbon nanoparticles | NCT05167149 | - | Sentinel lymph node (SLN) biopsy | [41] |
| Myocet (Cephalon) | Approved | Cancer Therapy |
Nanoimaging agents in pre-clinic and clinical trials
Nanotechnology has indeed revolutionized the biomedical arena. In contrast to the bulk materials, nanoparticles of the same material in the size regime of 10-9 m exhibit unique physicochemical properties. Their size, shape, and surface properties are highly tunable [21]. The large surface area to volume ratio enables the interaction of the nanoparticles with the subcellular structures. The accumulation of the nanoparticles at the diseased site, either by passive or active targeting, aids in theragnostic applications [7]. Moreover, unlike bulk materials, the physicochemical properties of nanoparticles are governed by the laws of quantum mechanics [22].
In biomedical research, several imaging modalities allow early diagnosis of diseases based on underlying pathophysiological conditions. Among these, X-ray, ultrasound, computed tomography, nuclear medicine, and magnetic resonance imaging are extensively used. The diagnostic efficacy in terms of sensitivity and specificity of these techniques can be improved several folds by using highly efficient contrast agents and targeting ligands. The conventionally used exogenous contrasts, such as those based on iodine, barium sulfate, and Gadolinium ions (Gd3+) complexes have limitations such as renal toxicity, fast clearance, etc., which prevent their extensive use [23]. This is where nanotechnology and nanoparticles revolutionize molecular imaging. The resolution and specificity can be increased several folds by using nano-contrast agents. The nano-contrast agents also exhibit high photostability, tunable surface chemistry, biocompatibility, targeting capability, etc. [24].
Over the past several years, there has been an explosion in the number and type of nanoparticles proposed for theragnostic applications. Table 1 shows some of the nano-contrast agents in the clinical trial.
To date, several types of nanoparticles have been developed and studied for theragnostic applications, such as micelles, liposomes, dendrimers, carbon nanotubes, metallic nanoparticles, quantum dots, etc. [25]. Recent research focuses on the engineering of nano-contrast agents which can respond to stimuli such as pH, redox potential, hypoxia, enzymes, or application of ultrasound, light, heat, and magnetic field [26]. Stimuli-sensitive nano-contrast agents further enhance the sensitivity and specificity of diagnosis, which has immense applications in precision medicine [27].
The past decade has witnessed extensive graphene use to prepare different nanoparticle types [28]. Graphene quantum dots (2-8 nm size) exhibiting unique physicochemical properties and bright red-luminescent graphene quantum dots can be prepared via a simple one-pot microwave-assisted green-synthesis route using ethanolic extracts of Mangifera indica (mango) leaves. They exhibit excitation-independent fluorescence emission in the near-infrared (NIR) region between 650 and 750 nm. These quantum dots are also biocompatible and demonstrate excellent uptake in cells and localize in the cytoplasm, enabling them to be used as NIR-responsive fluorescent bioimaging probes [29]. Graphene quantum dots were prepared using grape seed extract as a green therapeutic carbon source self-assembled in the aqueous medium. These quantum dots undergo localization in the nucleus, which enables imaging of the nucleus [30]. Bright multi-fluorescent carbon dots (C-dots) with size 3.3 ± 0.4 nm synthesized via prolonged microwave-assisted heating (PT-MAH) method demonstrate 'turn-on' fluorescence when bound to GSH. These C-dots exhibit excitation-dependent fluorescence that could be utilized for multicolor in vitro cellular imaging [31].
Liposomes are one of the extensively used nanoparticle platforms for theragnostic applications [32]. Liposomes modified with folic acid targeting ligands can be loaded with graphene quantum dots for in vivo tumor diagnosis due to their high contrast and emissive nature [33]. Hydroxypropyl-beta-cyclodextrin (CD) is an FDA-approved molecule for delivering lipophilic drugs to humans, and the loading of IR780 Dye in cyclodextrin nanoparticles allows specific imaging of atherosclerotic plaques [34].
Metallic nanoparticles and nanoclusters exhibit unique physicochemical properties because of the quantum confinement at the nanoscale [35]. Copper nanoclusters with lysozyme (Lys) as the stabilizing agent exhibit pH-dependent fluorescent properties. They are highly biocompatible and exhibit green fluorescence at neutral pH (normal physiological conditions), enabling cell imaging [36]. Albumin-mediated reduction and synthesis of gold nanostars produce highly stable plasmonic nanostructures with excellent biocompatibility. They also exhibit enhanced computed tomographic (C.T.) contrast suitable for in vivo diagnostic imaging [37].
The selection of nano-contrast agents for theragnostic applications depends on several factors, such as size, shape, surface charge, and microstructures, which depend on synthesis methods. Moreover, the nature of the disease, expression of the surface proteins, anatomical site, etc., should be considered while selecting the nano-contrast agent. Although considerable studies point out their potential applications in cost-effective and safe personalized medicine, significant concerns have been raised over their safety owing to the high reactivity of the nanoparticles. Hence further in-depth studies are warranted to assess their potential risks to health, the environment, and society.
Patentability challenges
A clear definition on the types of diagnostics and therapeutic constitutes yet to be decided prior to protect intellectual properties (IPs). Several components such as types of imaging and therapeutic probes, types of engineering systems and related approaches, product yield, types of surface modifiers, involved techniques, etc. can be protected during IP filing. However, the multistep engineering approaches which also owned by various companies or startups make IP process complicated or confusing. Overall, multiple patents association, institutes and cross-licensing agreements complicate the IP process. Hence, there is a need of new IP practices and related process which simplify the whole process of laboratory invention to commercialization which also reduce the involved expense and time along with fruitful licensing agreements and collaborations. It should be noted that IPs related to nanoimaging, nanotherapuetics, nanomedicine, nanotheranostics and nanotechnology, nanodiagnostics need to be peer reviewed by well trained and expert patent examiners. On the other hand, various regulatory factors and government policies need to be re-evaluate for easy commercialization of nanotechnology related products. To the best of our experience and knowledge, national and international collaborations between academia and industry, and regulatory agencies smooth the commercialization and its process. For example, lack of clear understanding on (i) developing and engineering diagnostics or therapuetics modality, (ii) regulatory guidelines, (iii) clinical trial requirements and design, (iv) imaging and treatment planning in patients lower the translational possibilities. It has been noticed that lab scale engineering medicines or diagnostics kits and their testing with pre-clinical models are always promising, but large-scale production and clinical testing are always challenging which also hamper the translational of laboratory technology. We should mention that large scale manufacturing also faces potential challenges related to lack of infrastructure and in-house expertise, side-products contaminations, poor quality control, complexities, high costs, etc. Especially nanoimaging and therapeutics engineering face major issues of large-scale good manufacturing (GMP) production. Some nanoengineered particulates fall under the regulatory purview of both drug and diagnostic agencies, hence, their regulatory landscape can be complex because different regulatory bodies have different requirements for approval and commercialization. Overall, nanoparticle-based IP process and obtaining their regulatory approvals can be time-consuming and expensive, which could hamper clinical translation and commercialization.
Conclusion and Perspectives
Biomedical, translational research is multifactorial and multi-disciplinary. A partnership between industry and academia is beneficial and should be adopted to reduce resources, developmental time, and cost. With the practice of several such translational works, Nanobios lab has created many successful examples over the last fifteen years in the Indian subcontinent and the world. The works of Prof Rohit Srivastava have been briefly identified and highlighted in this article to inspire researchers across the globe towards utilizing resources judiciously yet delivering high-value, affordable healthcare technologies.
Acknowledgements
The authors acknowledge Sophisticated Analytical Instrumentation Facility (SAIF) at IIT Bombay for instrumentation support. This work was supported by the Department of Biotechnology, Government of India. We are grateful to our collaborators and partners. Dr. Rajendra Prasad thanks the director and the school of biochemical engineering, IIT-BHU.
Competing Interests
R.S and R.P. are inventors of nanoparticle based on national and international patents. All authors declare no competing results that have appeared to influence the publishing of this manuscript and have given approval for final submission.
References
1. Ghare MMG, Umarani NS. Industry-academia Interaction-Need of the Hour. JournalNX. 2020:75-84
2. McPhee C, Hoppe M, Lindhult E. Action Research. Technology Innovation Management Review. 2019;9(4):3-5
3. Alvesson M, Sandberg J. Generating Research Questions Through Problematization. Academy of Management Review. 2011;36(2):247-271
4. Adler N, Styhre A. Collaborative research in organizations: Foundations for learning, change, and theoretical development. 2004. Sage.
5. Coughlan P, Coghlan D. Action research for operations management. International journal of operations & production management. 2002
6. Ellström P-E, Elg M, Wallo A, Berglund M, Kock H. Interactive research: concepts, contributions and challenges. Journal of Manufacturing Technology Management. 2020;31(8):1517-1537
7. Madamsetty VS, Mukherjee A, Mukherjee S. Recent Trends of the Bio-Inspired Nanoparticles in Cancer Theranostics. Front Pharmacol. 2019;10. doi:10.3389/fphar. 2019 01264
8. Metselaar JM, Lammers T. Challenges in nanomedicine clinical translation. Drug Deliv Transl Res. 2020;10(3):721-725
9. Shan X, Gong X, Li J, Wen J, Li Y, Zhang Z. Current approaches of nanomedicines in the market and various stage of clinical translation. Acta Pharm Sin B. 2022;12(7):3028-3048
10. Younis MA, Tawfeek HM, Abdellatif AAH, Abdel-Aleem JA, Harashima H. Clinical translation of nanomedicines: Challenges, opportunities, and keys. Adv Drug Deliv Rev. 2022;181:114083
11. Hua S, de Matos MBC, Metselaar JM, Storm G. Current Trends and Challenges in the Clinical Translation of Nanoparticulate Nanomedicines: Pathways for Translational Development and Commercialization. Front Pharmacol. 2018;9. doi:10.3389/fphar. 2018 00790
12. Bhattacharjee S, Brayden DJ. Addressing the challenges to increase the efficiency of translating nanomedicine formulations to patients. Expert Opin Drug Discov. 2021;16(3):235-254
13. Parhizkar M, Mahalingam S, Homer-Vanniasinkam S, Edirisinghe M. Latest developments in innovative manufacturing to combine nanotechnology with healthcare. Nanomedicine. 2018;13(1):5-8
14. Choi YH, Han H-K. Nanomedicines: current status and future perspectives in aspect of drug delivery and pharmacokinetics. J Pharm Investig. 2018;48(1):43-60
15. Sun D, Zhou S, Gao W. What Went Wrong with Anticancer Nanomedicine Design and How to Make It Right. ACS Nano. 2020;14(10):12281-12290
16. Hertig JB, Shah VP, Flühmann B, Mühlebach S, Stemer G, Surugue J. et al. Tackling the challenges of nanomedicines: are we ready? American Journal of Health-System Pharmacy. 2021;78(12):1047-1056
17. SEER (NIH). Cancer Stat Facts: Cancer of Any Site. 2022. https://seer.cancer.gov/statfacts/html/all.html
18. Cancer Research UK. Why is early diagnosis important? 2021. https://www.cancerresearchuk.org/about-cancer/cancer-symptoms/why-is-early-diagnosis-important
19. Davidson KW, Barry MJ, Mangione CM, Cabana M, Caughey AB, Davis EM. et al. Screening for Prediabetes and Type 2 Diabetes: US Preventive Services Task Force Recommendation Statement. JAMA - Journal of the American Medical Association. 2021;326(8):736-743
20. SCIE. Why early diagnosis of dementia is important. Social Care Institute for Excellence. 2020. https://www.scie.org.uk/dementia/symptoms/diagnosis/early-diagnosis.asp
21. Liu L, Liu R, Wang X, Cui Q, Yao C, Zhu S. et al. Facile Preparation of Fluorescent Nanoparticles with Tunable Exciplex Emission and Their Application to Targeted Cellular Imaging. ACS Appl Bio Mater. 2018;1(1):185-192
22. Sim S, Wong N. Nanotechnology and its use in imaging and drug delivery (Review). Biomed Rep. 2021;14(5):42
23. Naseri N, Ajorlou E, Asghari F, Pilehvar-Soltanahmadi Y. An update on nanoparticle-based contrast agents in medical imaging. Artif Cells Nanomed Biotechnol. 2018;46(6):1111-1121
24. Thakor AS, Jokerst J v, Ghanouni P, Campbell JL, Mittra E, Gambhir SS. Clinically Approved Nanoparticle Imaging Agents. Journal of Nuclear Medicine. 2016;57(12):1833-1837
25. Pallares RM, Mottaghy FM, Schulz V, Kiessling F, Lammers T. Nanoparticle Diagnostics and Theranostics in the Clinic. Journal of Nuclear Medicine. 2022;63(12):1802-1808
26. Mi P. Stimuli-responsive nanocarriers for drug delivery, tumor imaging, therapy and theranostics. Theranostics. 2020;10(10):4557-4588
27. Wang X, Zhong X, Lei H, Yang N, Gao X, Cheng L. Tumor microenvironment-responsive contrast agents for specific cancer imaging: a narrative review. J BioX Res. 2020;3(4):144-156
28. Lin J, Chen X, Huang P. Graphene-based nanomaterials for bioimaging. Adv Drug Deliv Rev. 2016;105:242-254
29. Kumawat MK, Thakur M, Gurung RB, Srivastava R. Graphene Quantum Dots from Mangifera indica: Application in Near-Infrared Bioimaging and Intracellular Nanothermometry. ACS Sustain Chem Eng. 2017;5(2):1382-1391
30. Kumawat MK, Thakur M, Gurung RB, Srivastava R. Graphene Quantum Dots for Cell Proliferation, Nucleus Imaging, and Photoluminescent Sensing Applications. Sci Rep. 2017;7(1):15858
31. Borse V, Thakur M, Sengupta S, Srivastava R. N-doped multi-fluorescent carbon dots for 'turn off-on' silver-biothiol dual sensing and mammalian cell imaging application. Sens Actuators B Chem. 2017;248:481-492
32. Lee W, Im H-J. Theranostics Based on Liposome: Looking Back and Forward. Nucl Med Mol Imaging. 2019;53(4):242-246
33. Prasad R, Jain NK, Yadav AS, Chauhan DS, Devrukhkar J, Kumawat MK. et al. Liposomal nanotheranostics for multimode targeted in vivo bioimaging and near-infrared light mediated cancer therapy. Commun Biol. 2020;3(1):284
34. Mehta S, Bongcaron V, Nguyen TK, Jirwanka Y, Maluenda A, Walsh APG. et al. An Ultrasound-Responsive Theranostic Cyclodextrin-Loaded Nanoparticle for Multimodal Imaging and Therapy for Atherosclerosis. Small. 2022;18(31):2200967
35. Chen D, Monteiro-Riviere NA, Zhang LW. Intracellular imaging of quantum dots, gold, and iron oxide nanoparticles with associated endocytic pathways. WIREs Nanomedicine and Nanobiotechnology. 2017 9(2). doi:10.1002/wnan.1419
36. Thawari AG, Kumar P, Srivastava R, Rao CP. Lysozyme coated copper nanoclusters for green fluorescence and their utility in cell imaging. Mater Adv. 2020;1(5):1439-1447
37. Sasidharan S, Bahadur D, Srivastava R. Rapid, One-Pot, Protein-Mediated Green Synthesis of Gold Nanostars for Computed Tomographic Imaging and Photothermal Therapy of Cancer. ACS Sustain Chem Eng. 2017;5(11):10163-10175
38. Phillips E, Penate-Medina O, Zanzonico PB, Carvajal RD, Mohan P, Ye Y. et al. Clinical translation of an ultrasmall inorganic optical-PET imaging nanoparticle probe. Sci Transl Med. 2014 6(260). doi:10.1126/scitranslmed.3009524
39. Richards JMJ, Shaw CA, Lang NN, Williams MC, Semple SIK, MacGillivray TJ. et al. In Vivo Mononuclear Cell Tracking Using Superparamagnetic Particles of Iron Oxide. Circ Cardiovasc Imaging. 2012;5(4):509-517
40. Zanoni DK, Stambuk HE, Madajewski B, Montero PH, Matsuura D, Busam KJ. et al. Use of Ultrasmall Core-Shell Fluorescent Silica Nanoparticles for Image-Guided Sentinel Lymph Node Biopsy in Head and Neck Melanoma. JAMA Netw Open. 2021;4(3):e211936
41. Zuo J, Wu LY, Cheng M, Bai P, Lei CZ, Li N. et al. Comparison Study of Laparoscopic Sentinel Lymph Node Mapping in Endometrial Carcinoma Using Carbon Nanoparticles and Lymphatic Pathway Verification. J Minim Invasive Gynecol. 2019;26(6):1125-1132
42. Rengan AK, Bukhari AB, Pradhan A, Malhotra R, Banerjee R, Srivastava R. et al. In vivo analysis of biodegradable liposome gold nanoparticles as efficient agents for photothermal therapy of cancer. Nano Lett. 2015 15(2). doi:10.1021/nl5045378
43. Kapse-Mistry S, Govender T, Srivastava R, Yergeri M. Nanodrug delivery in reversing multidrug resistance in cancer cells. Frontiers in Pharmacology. 2014;5 JUL. doi:10.3389/fphar. 2014 00159
Author Biography
 Prof. Rohit Srivastava is currently working as a Himanshu Patel Chair Professor at the Department of Biosciences and Bioengineering (BSBE), Indian Institute of Technology, (IIT) Bombay. Prof Srivastava completed his B.E. in Electronics Engineering from VNIT Nagpur in 1999 with distinction and was the recipient of a scholarship of merit for graduating students for the year. He joined Tata Consultancy Services, SEEPZ, Mumbai for a short duration of a year. Not finding satisfaction in what he was doing, he decided to complete higher studies at U.S. He subsequently went on to finish a Master of Science and a Ph.D. in Biomedical Engineering from Louisiana Tech University, Ruston, LA, USA for which he received the best student of the year award in 2005. His specialization lies in POC Diagnostic devices, Biomedical Microsystems devices (MEMS), nanoengineered biosensors, photothermal therapy in cancers and nanoengineered orthopedic applications. His lab has graduated 35 Ph.D. students and 80 Master's students at IIT Bombay. Currently, his lab at IIT Bombay comprises 22 PhD students, 10 Master's students, 5 post-docs, 15 interns and 3 staff. His lab has funded projects across all domains, including point-of-care diagnostic devices, biosensors, cancer nanotechnology, MEMS drug delivery devices, and orthopedic solutions. To date, Prof. Srivastava has obtained a h-index of 41 and an i10-index of 158 with 6717 citations in reputed journals. He is an active contributor to the translational research community with a focus on drug delivery, cancer theranostics and other domains of nanobiotechnology. He has authored and contributed to more than 300 publications, including research papers, review articles, books, and book chapters. His three most cited publications include (a) In vivo analysis of biodegradable liposome gold nanoparticles as effectual agents for photothermal therapy of cancer (cited 340 times) [42], (b) Nano-drug delivery in reversing multidrug resistance in cancer cells (Cited 291 times) [43], (c) Graphene Quantum Dots from Mangifera indica: Application in Near-Infrared Bioimaging and Intracellular Nanothermometry (cited 229 times) [29]. Prof Rohit Srivastava has been granted a total of 30 patents (including 3 U.S. patents and 27 Indian patents), and currently has over 150+ IDFs, Trademarks, Design Registrations, patents filed or in the process of being filed.
Prof. Rohit Srivastava is currently working as a Himanshu Patel Chair Professor at the Department of Biosciences and Bioengineering (BSBE), Indian Institute of Technology, (IIT) Bombay. Prof Srivastava completed his B.E. in Electronics Engineering from VNIT Nagpur in 1999 with distinction and was the recipient of a scholarship of merit for graduating students for the year. He joined Tata Consultancy Services, SEEPZ, Mumbai for a short duration of a year. Not finding satisfaction in what he was doing, he decided to complete higher studies at U.S. He subsequently went on to finish a Master of Science and a Ph.D. in Biomedical Engineering from Louisiana Tech University, Ruston, LA, USA for which he received the best student of the year award in 2005. His specialization lies in POC Diagnostic devices, Biomedical Microsystems devices (MEMS), nanoengineered biosensors, photothermal therapy in cancers and nanoengineered orthopedic applications. His lab has graduated 35 Ph.D. students and 80 Master's students at IIT Bombay. Currently, his lab at IIT Bombay comprises 22 PhD students, 10 Master's students, 5 post-docs, 15 interns and 3 staff. His lab has funded projects across all domains, including point-of-care diagnostic devices, biosensors, cancer nanotechnology, MEMS drug delivery devices, and orthopedic solutions. To date, Prof. Srivastava has obtained a h-index of 41 and an i10-index of 158 with 6717 citations in reputed journals. He is an active contributor to the translational research community with a focus on drug delivery, cancer theranostics and other domains of nanobiotechnology. He has authored and contributed to more than 300 publications, including research papers, review articles, books, and book chapters. His three most cited publications include (a) In vivo analysis of biodegradable liposome gold nanoparticles as effectual agents for photothermal therapy of cancer (cited 340 times) [42], (b) Nano-drug delivery in reversing multidrug resistance in cancer cells (Cited 291 times) [43], (c) Graphene Quantum Dots from Mangifera indica: Application in Near-Infrared Bioimaging and Intracellular Nanothermometry (cited 229 times) [29]. Prof Rohit Srivastava has been granted a total of 30 patents (including 3 U.S. patents and 27 Indian patents), and currently has over 150+ IDFs, Trademarks, Design Registrations, patents filed or in the process of being filed.
![]() Corresponding authors: Rohit Srivastava, Department of Biosciences and Bioengineering, Indian Institute of Technology, Bobay, Powai, 76001, India. Email: rsrivastaac.in. Rajendra Prasad; School of Biochemical Engineering, Indian Institute of Technology (BHU), Varanasi 221005, India. Email: rajendra.bceac.in.
Corresponding authors: Rohit Srivastava, Department of Biosciences and Bioengineering, Indian Institute of Technology, Bobay, Powai, 76001, India. Email: rsrivastaac.in. Rajendra Prasad; School of Biochemical Engineering, Indian Institute of Technology (BHU), Varanasi 221005, India. Email: rajendra.bceac.in.

 Global reach, higher impact
Global reach, higher impact